
The molecular geometry of \[B{F_3}\] is:
A. Tetrahedral
B. Pyramidal
C. Square planar
D. Trigonal planar
Answer
564.3k+ views
Hint: The molecular geometry depends on the hybridization of the central atom. It also depends on the number of bond pairs and lone pairs of electrons present on the central atom.
Complete step by step answer: The given compound is boron trifluoride. It is an inorganic compound which consists of boron and fluorine elements. Boron is the central atom which is bonded to three fluorine atoms as boron is the least electronegative of the two elements.
Boron is an element in the periodic table with atomic number \[5\] and electronic configuration \[\left[ {He} \right]2{s^2}2{p^1}\] . Thus the valence shell of boron is \[2\] and contains three electrons of which two are in \[2s\] and one in \[2p\] orbital.
The number of bonded fluorine atoms with the central boron atom is three. Thus the VSEP number is equal to = \[\dfrac{1}{2}\] (number of valence electrons of central boron atom + number of electrons shared by other bonded fluorine atoms)
$ = \dfrac{1}{2}(3 + 3) = 3$
The VSEP number equal to \[3\] belongs to \[s{p^2}\] hybridization. The shape or geometry of the molecule is trigonal planar. The bond angles between the \[F - B - F\] bond is \[120^\circ \] . The structure of the molecule is
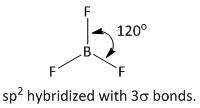
Thus option D is the correct answer..
Note:The rules for predicting the shapes of the molecules are: a) identify the central atom (the bonded atom which is the least electronegative), b) count the number of valence electrons of central atom, c) count the number of electrons shared by other bonded atoms, d) sum up the total of b and c and divide by two gives the VSEP number. The VSEP number corresponds to shape of the molecule as \[2\] for linear shape, \[3\] for trigonal planar, \[4\] for tetrahedral, \[5\] for trigonal bipyramidal, \[6\] for octahedral, and \[7\] for pentagonal bipyramidal.
Complete step by step answer: The given compound is boron trifluoride. It is an inorganic compound which consists of boron and fluorine elements. Boron is the central atom which is bonded to three fluorine atoms as boron is the least electronegative of the two elements.
Boron is an element in the periodic table with atomic number \[5\] and electronic configuration \[\left[ {He} \right]2{s^2}2{p^1}\] . Thus the valence shell of boron is \[2\] and contains three electrons of which two are in \[2s\] and one in \[2p\] orbital.
The number of bonded fluorine atoms with the central boron atom is three. Thus the VSEP number is equal to = \[\dfrac{1}{2}\] (number of valence electrons of central boron atom + number of electrons shared by other bonded fluorine atoms)
$ = \dfrac{1}{2}(3 + 3) = 3$
The VSEP number equal to \[3\] belongs to \[s{p^2}\] hybridization. The shape or geometry of the molecule is trigonal planar. The bond angles between the \[F - B - F\] bond is \[120^\circ \] . The structure of the molecule is

Thus option D is the correct answer..
Note:The rules for predicting the shapes of the molecules are: a) identify the central atom (the bonded atom which is the least electronegative), b) count the number of valence electrons of central atom, c) count the number of electrons shared by other bonded atoms, d) sum up the total of b and c and divide by two gives the VSEP number. The VSEP number corresponds to shape of the molecule as \[2\] for linear shape, \[3\] for trigonal planar, \[4\] for tetrahedral, \[5\] for trigonal bipyramidal, \[6\] for octahedral, and \[7\] for pentagonal bipyramidal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




