
The molecular formula of sodium carbonate is:
(A) \[{\text{NaC}}{{\text{O}}_3}\]
(B) \[\left( {{\text{N}}{{\text{H}}_4}} \right){\text{S}}{{\text{O}}_4}\]
(C) \[{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}\]
(D) \[{\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4}\]
Answer
591.6k+ views
Hint: First determine the charge on the cation and the anion. Then apply criss cross method to obtain the number of cations and anions. Then write the chemical symbols of various elements / polyatomic ions and use subscripts to indicate the number of each element / polyatomic ion.
Complete answer:
Sodium carbonate is the salt of weak acid carbonic acid with strong base sodium hydroxide. Carbonic acid is dibasic acid. Sodium carbonate contains sodium cations and carbonate anions. Sodium cation has unit positive charge and can be represented as \[{\text{N}}{{\text{a}}^ + }\]. Carbonate anion has two negative charges and can be represented as \[{\text{CO}}_3^{2 - }\]. The formula of sodium carbonate can be obtained by applying the criss cross method.
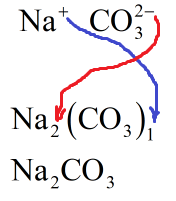
The charge on sodium cation is +1. Hence, the number of carbonate ions will be one. The charge on carbonate ions is -2. Hence, there will be two sodium cations.
The molecular formula of sodium carbonate is \[{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}\] :
Hence, the correct option is the option (D).
Additional Information: You can check the correctness of a molecular formula by calculating the total positive charges and total negative charges. One sodium cation has unit positive charge and two sodium cations have total positive charges of +2. The charge on the carbonate ion is -2. So the magnitude of total positive charge is equal to the magnitude of total negative charge. Hence, the molecule is electrically neutral.
The molecular formula \[{\text{NaC}}{{\text{O}}_3}\] is incorrect as the total number of positive charges is 1, whereas the total number of negative charges is -2. The electrical neutrality is not maintained.
The molecular formula \[{\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4}\] represents ammonium sulphate molecule. It contains ammonium cation \[\left( {{\text{NH}}_4^ + } \right)\] and sulphate anion \[\left( {{\text{SO}}_4^{2 - }} \right)\] .
Note: Ions can contain a single atom or several atoms. Examples of ions containing single atom are \[{\text{N}}{{\text{a}}^ + }{\text{, C}}{{\text{l}}^ - }\] . Polyatomic ions contain several atoms of the same or different elements. The examples of polyatomic ions include \[{\text{NH}}_4^ + ,{\text{ CO}}_3^{2 - }{\text{, SO}}_4^{2 - }\] etc.
Complete answer:
Sodium carbonate is the salt of weak acid carbonic acid with strong base sodium hydroxide. Carbonic acid is dibasic acid. Sodium carbonate contains sodium cations and carbonate anions. Sodium cation has unit positive charge and can be represented as \[{\text{N}}{{\text{a}}^ + }\]. Carbonate anion has two negative charges and can be represented as \[{\text{CO}}_3^{2 - }\]. The formula of sodium carbonate can be obtained by applying the criss cross method.

The charge on sodium cation is +1. Hence, the number of carbonate ions will be one. The charge on carbonate ions is -2. Hence, there will be two sodium cations.
The molecular formula of sodium carbonate is \[{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}\] :
Hence, the correct option is the option (D).
Additional Information: You can check the correctness of a molecular formula by calculating the total positive charges and total negative charges. One sodium cation has unit positive charge and two sodium cations have total positive charges of +2. The charge on the carbonate ion is -2. So the magnitude of total positive charge is equal to the magnitude of total negative charge. Hence, the molecule is electrically neutral.
The molecular formula \[{\text{NaC}}{{\text{O}}_3}\] is incorrect as the total number of positive charges is 1, whereas the total number of negative charges is -2. The electrical neutrality is not maintained.
The molecular formula \[{\left( {{\text{N}}{{\text{H}}_4}} \right)_2}{\text{S}}{{\text{O}}_4}\] represents ammonium sulphate molecule. It contains ammonium cation \[\left( {{\text{NH}}_4^ + } \right)\] and sulphate anion \[\left( {{\text{SO}}_4^{2 - }} \right)\] .
Note: Ions can contain a single atom or several atoms. Examples of ions containing single atom are \[{\text{N}}{{\text{a}}^ + }{\text{, C}}{{\text{l}}^ - }\] . Polyatomic ions contain several atoms of the same or different elements. The examples of polyatomic ions include \[{\text{NH}}_4^ + ,{\text{ CO}}_3^{2 - }{\text{, SO}}_4^{2 - }\] etc.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




