
The molecular formula of heavy water is:
a.) \[{{D}_{3}}O\]
b.) \[{{D}_{2}}O\]
c.) \[DO\]
d.) \[D{{O}_{2}}\]
Answer
601.8k+ views
Hint: The formula of heavy water is the same as that of normal water. This is because oxygen has six valence electrons and can covalently bond as long as its valency is satisfied. Deuterium is an isotope of hydrogen.
Complete step by step answer:
Deuterium (D) is an isotope of hydrogen. It is present in very low quantities in the environment. It is around 0.0156 percent of the total environmental oxygen. It contains one neutron and one proton. It is called ‘deuterium’ because it has double the mass of protium, it weighs around 2.014 u. For the same reason, it is also known as heavy hydrogen.
And therefore, water made by deuterium is known as heavy water. It is the same as normal water, the difference being, its mass is 10 percent more than normal water.
Water is a covalent compound, formed by sharing of electrons between oxygen and two hydrogen (deuterium) atoms. Oxygen, having 6 electrons in its valence, forms two bonds with deuterium. Therefore, the formula of heavy water is \[{{D}_{2}}O\]. The structure can be represented as –
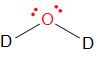 Therefore, the answer is – option (b) – The molecular formula of heavy water is \[{{D}_{2}}O\].
Therefore, the answer is – option (b) – The molecular formula of heavy water is \[{{D}_{2}}O\].
Additional Information:
There are seven isotopes of hydrogen, i.e. Hydrogen-1 (protium), Hydrogen-2 (deuterium), Hydrogen-3 (tritium), Hydrogen-4, Hydrogen-5, Hydrogen-6, Hydrogen-7.
Note: The other two main isotopes of hydrogen are – protium and tritium.
Protium – It is the hydrogen which occurs in the periodic table. It is also known as atomic hydrogen. It contains one neutron and one proton. It is very stable. 99.9 percent of hydrogen present in the environment is protium.
Tritium – This isotope of hydrogen contains two neutrons and one proton. It is quite unstable, with a half-life of 12.32 years.
Complete step by step answer:
Deuterium (D) is an isotope of hydrogen. It is present in very low quantities in the environment. It is around 0.0156 percent of the total environmental oxygen. It contains one neutron and one proton. It is called ‘deuterium’ because it has double the mass of protium, it weighs around 2.014 u. For the same reason, it is also known as heavy hydrogen.
And therefore, water made by deuterium is known as heavy water. It is the same as normal water, the difference being, its mass is 10 percent more than normal water.
Water is a covalent compound, formed by sharing of electrons between oxygen and two hydrogen (deuterium) atoms. Oxygen, having 6 electrons in its valence, forms two bonds with deuterium. Therefore, the formula of heavy water is \[{{D}_{2}}O\]. The structure can be represented as –

Additional Information:
There are seven isotopes of hydrogen, i.e. Hydrogen-1 (protium), Hydrogen-2 (deuterium), Hydrogen-3 (tritium), Hydrogen-4, Hydrogen-5, Hydrogen-6, Hydrogen-7.
Note: The other two main isotopes of hydrogen are – protium and tritium.
Protium – It is the hydrogen which occurs in the periodic table. It is also known as atomic hydrogen. It contains one neutron and one proton. It is very stable. 99.9 percent of hydrogen present in the environment is protium.
Tritium – This isotope of hydrogen contains two neutrons and one proton. It is quite unstable, with a half-life of 12.32 years.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




