
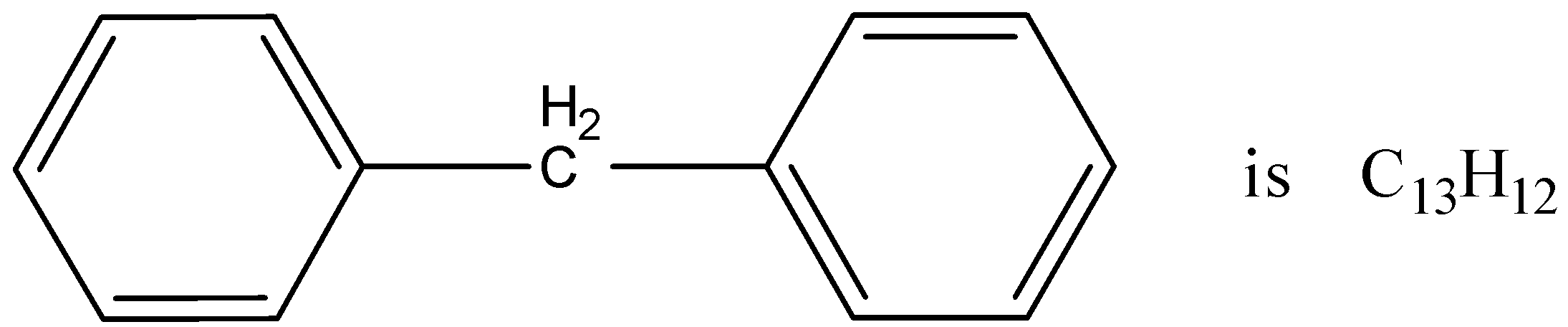
The molecular formula of diphenylmethane is ${C_{13}}{H_{12}}$. How many structural isomers are possible when one of the hydrogens is replaced by a chlorine atom?
A. $8$
B. $7$
C. $6$
D. $4$

Answer
547.5k+ views
Hint: There are carbon molecules which can form structural isomers because of the presence of the different residues associated with the main structures or the functional groups in the compound. Here any of the $H$ can be replaced which means all the isomers are going to be positional isomers of $Cl$. Since there are two benzene rings the position in each of the benzene getting replaced replicates the chemical nature of similar isomers.
Complete step by step answer:
The molecular formula of the chemical molecule that is given here is ${C_{13}}{H_{12}}$ and the chemical structure is known diphenylmethane. This chemical compound is known as diphenylmethane as there is a methyl residue in the middle and there are two benzene ring structures associated with the central carbon atom. If one of the $H$ residue is replaced by $Cl$ residue from the whole diphenyl methane structure then there can be different structural isomers according to the position of the $Cl$ residue inside the main structure.
The first isomer can be formed if any of the hydrogen in the central carbon of the methyl residue is replaced by chloride bond formation. There can be other three positional isomers which can be based on the positioning of the chloride residue on the benzene structure. There can be chloride at the ortho-position, para-position and meta-position. All of these positional isomers prove that there can be four possible isomers if one of the $H$ is replaced by $Cl$.
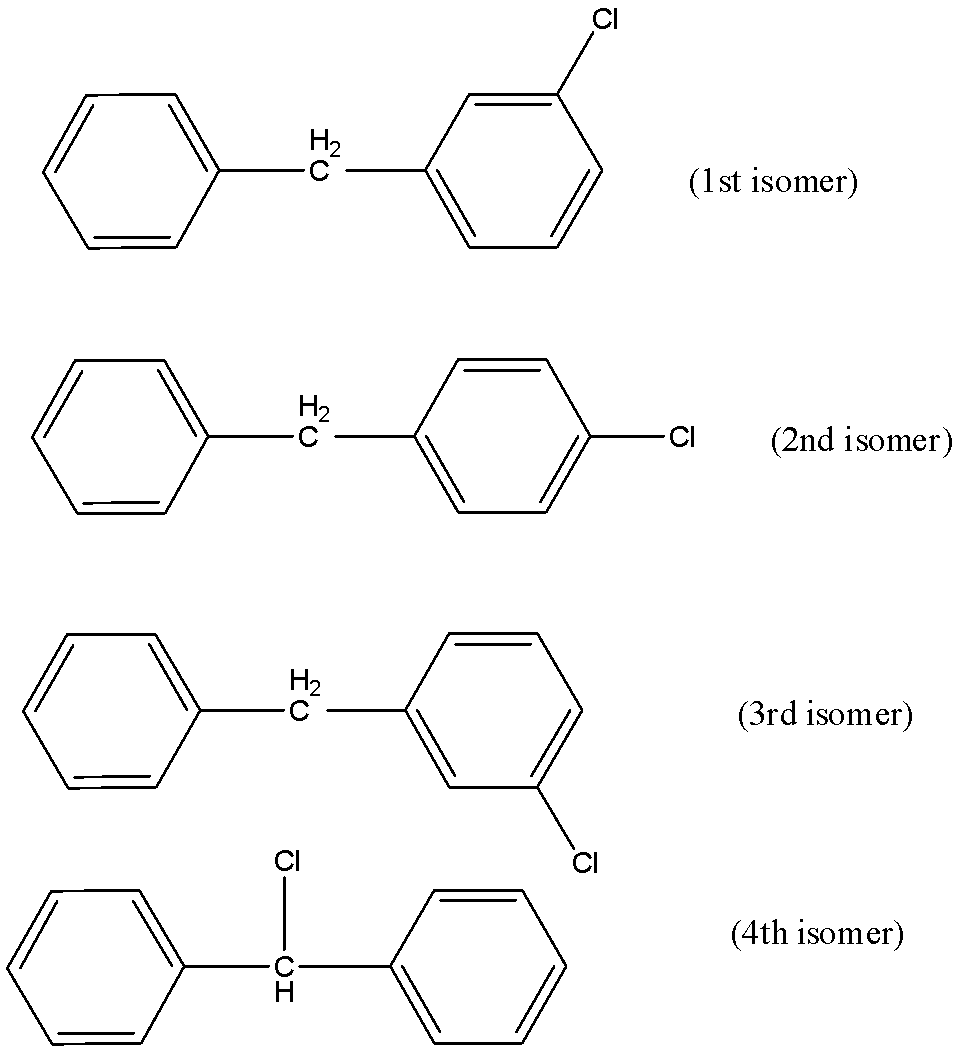
These are the $4$ different positional isomers that can be produced.
Note: Different isomers can be formed based on the functional groups present. This can be based on the type of position of the specific functional group involved in the given molecule or the type of functional group present.
Complete step by step answer:
The molecular formula of the chemical molecule that is given here is ${C_{13}}{H_{12}}$ and the chemical structure is known diphenylmethane. This chemical compound is known as diphenylmethane as there is a methyl residue in the middle and there are two benzene ring structures associated with the central carbon atom. If one of the $H$ residue is replaced by $Cl$ residue from the whole diphenyl methane structure then there can be different structural isomers according to the position of the $Cl$ residue inside the main structure.
The first isomer can be formed if any of the hydrogen in the central carbon of the methyl residue is replaced by chloride bond formation. There can be other three positional isomers which can be based on the positioning of the chloride residue on the benzene structure. There can be chloride at the ortho-position, para-position and meta-position. All of these positional isomers prove that there can be four possible isomers if one of the $H$ is replaced by $Cl$.

These are the $4$ different positional isomers that can be produced.
Note: Different isomers can be formed based on the functional groups present. This can be based on the type of position of the specific functional group involved in the given molecule or the type of functional group present.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




