
The mass number of the sulphur atom is? (Atomic Number=16 and Number of Neutrons=16)
(A) 18
(B) 32
(C) 24
(D) 16
Answer
587.7k+ views
Hint: An atom is composed of electrons, protons and neutrons. Electrons are the negative charges, Protons are the positive charges and the neutrons are the neutral charges.
Complete step by step solution:
The mass number also called atomic mass number or nucleon number is the total number of protons and neutrons (together known as nucleons) in a nucleus. It is approximately equal to the atomic mass of the atom expressed in Dalton. The protons and neutrons are called baryons. Thus the total mass number A is the total no. of protons and neutrons of an atom or ion. The mass number is different for each different isotope of a chemical element. Hence, the difference between the mass number and the atomic number Z gives the number of neutrons (N) in a given nucleus: N = A − Z.
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus.
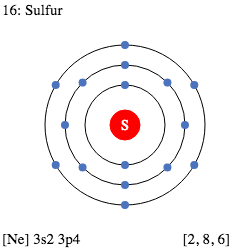
Thus for Sulphur,
N= 16
Z= 16
Thus, Mass number A= N+Z = 32 amu or Dalton.
The answer is (B).
Additional information:
Sulphur is a multivalent non-metal, abundant, tasteless and odourless. In its native form sulphur is a yellow crystalline solid. In nature, it occurs as the pure element or as sulfide and sulfate minerals.
Note: The number of protons and electrons are the same in any neutral atom. Thus, the atomic number is equal to the number of protons/electrons in a neutral atom.
Complete step by step solution:
The mass number also called atomic mass number or nucleon number is the total number of protons and neutrons (together known as nucleons) in a nucleus. It is approximately equal to the atomic mass of the atom expressed in Dalton. The protons and neutrons are called baryons. Thus the total mass number A is the total no. of protons and neutrons of an atom or ion. The mass number is different for each different isotope of a chemical element. Hence, the difference between the mass number and the atomic number Z gives the number of neutrons (N) in a given nucleus: N = A − Z.
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus.
Thus for Sulphur,
N= 16
Z= 16
Thus, Mass number A= N+Z = 32 amu or Dalton.
The answer is (B).
Additional information:
Sulphur is a multivalent non-metal, abundant, tasteless and odourless. In its native form sulphur is a yellow crystalline solid. In nature, it occurs as the pure element or as sulfide and sulfate minerals.

Note: The number of protons and electrons are the same in any neutral atom. Thus, the atomic number is equal to the number of protons/electrons in a neutral atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




