
The major product formed in the reaction of toluene with chlorine in the presence of ferric chloride is:
This question has multiple correct options
A. o-chlorotoluene
B. m-chlorotoluene
C. p-chlorotoluene
D. benzyl chloride
Answer
584.4k+ views
Hint: We know that in case of substituted aromatic compounds, the functional group present in the compound directs the next incoming group to a specific position in the aromatic ring. We call this as the directive influence of the group already bonded to the benzene ring.
Complete step by step answer:
We must remember that if a monosubstituted compound is treated with an electrophile, it will undergo electrophilic aromatic substitution reaction and form a disubstituted compound which may be recognized using the descriptors ortho, meta, and para. If the relative yield of the ortho product and the para products are greater than that of the meta product, then the substituent present in the monosubstituted ring is named an ortho, para directing group.
As we know that the electron donating groups are usually ortho/para directors for electrophilic aromatic substitutions, whereas electron withdrawing groups are usually meta directors. The halogens are ortho/para directors since they need lone pairs of electrons to share with the aromatic ring.
We have to remember that the electron donating group are referred to as activating groups, though steric effects can interfere with the reaction. An electron withdrawing group has the opposite effect on the nucleophilicity of the ring. The electron withdrawing group takes away electron density from a Pi system, thereby making it less reactive during this kind of reaction and thus called as deactivating groups.
When toluene reacts with chlorine in the presence of ferric chloride it gives o-chlorotoluene and para-toluene and hydrochloride as the products. The major products formed in this reaction are o-chlorotoluene and p-chlorotoluene.
We know that the methyl group present in the benzene ring is ortho and para directing. Since the methyl group activates the ring at the para and ortho position much more than the meta positions, the electrophilic substitution reactions occur at these positions. We can write the chemical reaction as,
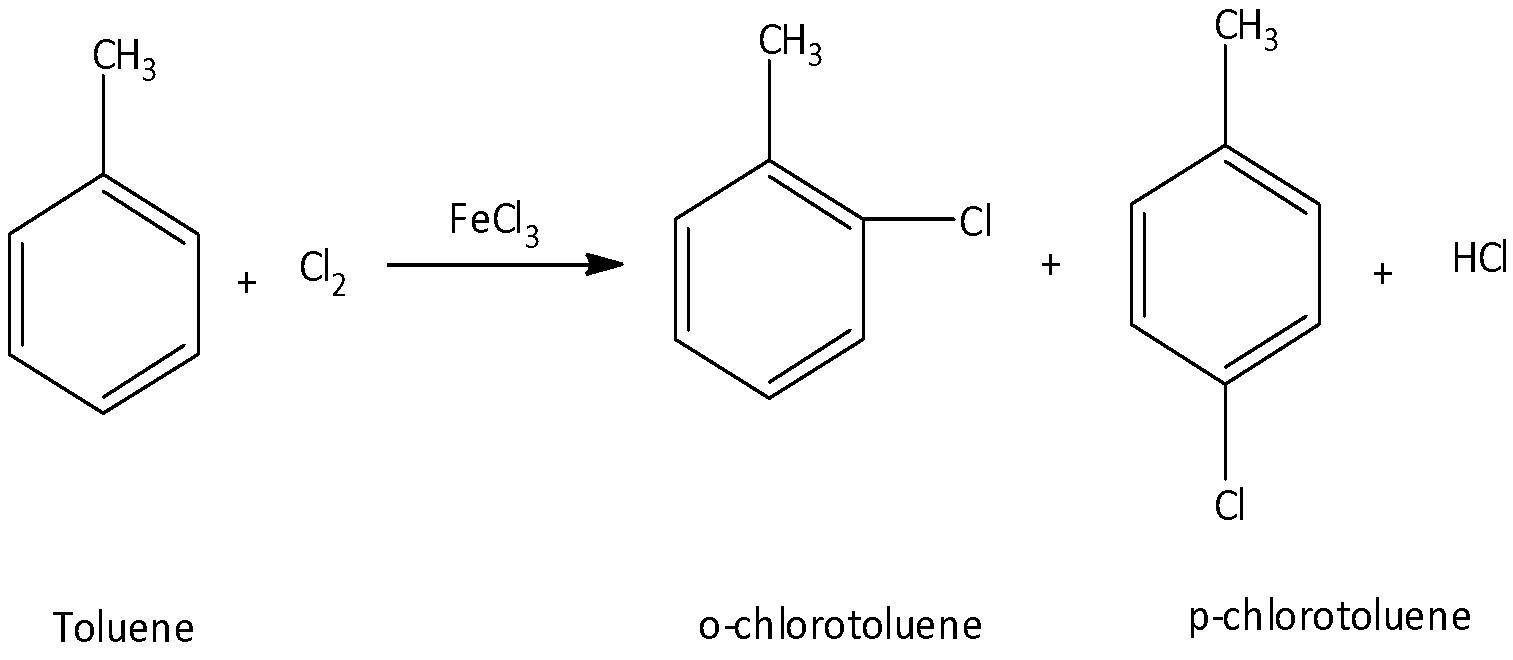
So, the correct answer is Option A,C.
Note: We must remember that if toluene reacts with chlorine in the absence of catalyst, the product would be trichloromethylbenzene. In this case, the substitution takes place at the methyl group rather than the ring. The presence of ortho and para directing group gives a stable cation only when the substituents enter the ortho and para position with respect to the substituent already present.
Complete step by step answer:
We must remember that if a monosubstituted compound is treated with an electrophile, it will undergo electrophilic aromatic substitution reaction and form a disubstituted compound which may be recognized using the descriptors ortho, meta, and para. If the relative yield of the ortho product and the para products are greater than that of the meta product, then the substituent present in the monosubstituted ring is named an ortho, para directing group.
As we know that the electron donating groups are usually ortho/para directors for electrophilic aromatic substitutions, whereas electron withdrawing groups are usually meta directors. The halogens are ortho/para directors since they need lone pairs of electrons to share with the aromatic ring.
We have to remember that the electron donating group are referred to as activating groups, though steric effects can interfere with the reaction. An electron withdrawing group has the opposite effect on the nucleophilicity of the ring. The electron withdrawing group takes away electron density from a Pi system, thereby making it less reactive during this kind of reaction and thus called as deactivating groups.
When toluene reacts with chlorine in the presence of ferric chloride it gives o-chlorotoluene and para-toluene and hydrochloride as the products. The major products formed in this reaction are o-chlorotoluene and p-chlorotoluene.
We know that the methyl group present in the benzene ring is ortho and para directing. Since the methyl group activates the ring at the para and ortho position much more than the meta positions, the electrophilic substitution reactions occur at these positions. We can write the chemical reaction as,

So, the correct answer is Option A,C.
Note: We must remember that if toluene reacts with chlorine in the absence of catalyst, the product would be trichloromethylbenzene. In this case, the substitution takes place at the methyl group rather than the ring. The presence of ortho and para directing group gives a stable cation only when the substituents enter the ortho and para position with respect to the substituent already present.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




