
The lightest radioactive isotope in periodic table is:
(A) tritium
(B) deuterium
(C) protium
(D) none of these
Answer
531.4k+ views
Hint: The lightest element in the periodic table is hydrogen and the radioactive form of hydrogen is determined by the nuclei with neutron to proton ratio as the regular hydrogen has zero neutrons.
Complete step by step answer:
The hydrogen having atomic number\[1\] and atomic mass\[1.0079\,amu\], has one proton and zero neutron in its nucleus, along with an electron in the shell/orbit which makes it the lightest element in the periodic table.
There are three naturally occurring isotopes (with same number of proton but different number of neutrons, thus varying mass number) of hydrogen:
Protium,\[({}_{1}^{1}\text{H)}\],Deuterium, \[({}_{1}^{2}\text{H)}\],Tritium, \[({}_{1}^{3}\text{H)}\]
The radioactive isotope are the elements with unstable nuclei, giving out atomic radiations in order to attain stability. During the emission, the element transforms into another chemical element or form of energy.
Out of the three isotopes, tritium is the most stable radioisotope among all the radioactive isotopes of hydrogen. Whereas, both protium and deuterium are non-radioactive stable particles.
The chemical properties of the isotopes of hydrogen are similar as they have the same electronic configuration.
Therefore option (A)- tritium is the lightest radioactive isotope in the periodic table.
Additional information: When in the water molecule the protium is replaced by deuterium, this water enriched in the molecule is called heavy water.
Note: The tritium radioisotope undergoes only beta-decay and changes into\[Helium-3\], with half-life of \[\text{12}\text{.32 years}\]. Where the neutron is converted into a proton by emission of electron (\[\beta -\text{particle}\]) and electron neutrino.
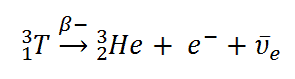
The four other heavier synthetic radioactive isotopes of hydrogen are (that is, \[({}_{1}^{4}\text{H)}\],\[({}_{1}^{5}\text{H)}\],\[({}_{1}^{6}\text{H)}\],\[({}_{1}^{7}\text{H)}\]) produced, but due to their extreme volatile nature. They do not exist.
Complete step by step answer:
The hydrogen having atomic number\[1\] and atomic mass\[1.0079\,amu\], has one proton and zero neutron in its nucleus, along with an electron in the shell/orbit which makes it the lightest element in the periodic table.
There are three naturally occurring isotopes (with same number of proton but different number of neutrons, thus varying mass number) of hydrogen:
Protium,\[({}_{1}^{1}\text{H)}\],Deuterium, \[({}_{1}^{2}\text{H)}\],Tritium, \[({}_{1}^{3}\text{H)}\]
The radioactive isotope are the elements with unstable nuclei, giving out atomic radiations in order to attain stability. During the emission, the element transforms into another chemical element or form of energy.
Out of the three isotopes, tritium is the most stable radioisotope among all the radioactive isotopes of hydrogen. Whereas, both protium and deuterium are non-radioactive stable particles.
The chemical properties of the isotopes of hydrogen are similar as they have the same electronic configuration.
Therefore option (A)- tritium is the lightest radioactive isotope in the periodic table.
Additional information: When in the water molecule the protium is replaced by deuterium, this water enriched in the molecule is called heavy water.
Note: The tritium radioisotope undergoes only beta-decay and changes into\[Helium-3\], with half-life of \[\text{12}\text{.32 years}\]. Where the neutron is converted into a proton by emission of electron (\[\beta -\text{particle}\]) and electron neutrino.
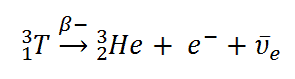
The four other heavier synthetic radioactive isotopes of hydrogen are (that is, \[({}_{1}^{4}\text{H)}\],\[({}_{1}^{5}\text{H)}\],\[({}_{1}^{6}\text{H)}\],\[({}_{1}^{7}\text{H)}\]) produced, but due to their extreme volatile nature. They do not exist.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




