
The lattice energy of $NaCl(s)$ using the following data will be:
Heat of sublimation of $Na(s) = S$
${(IE)_1}$ of $Na(g) = I$
Bond dissociation energy of $C{l_2}(g) = D$
Electron affinity of $Cl(g) = - E$
Heat of formation of $NaCl(s) = - Q$
A. Lattice energy$ - U = S + I + \dfrac{D}{2} - E - Q$
B. Lattice energy$ - U = S - I + \dfrac{D}{2} - E - Q$
C. Lattice energy$ - U = S + I + \dfrac{D}{2} + E - Q$
D. Lattice energy$ - U = S - I - \dfrac{D}{2} + E + Q$
Answer
584.1k+ views
Hint:The lattice energy is defined as the amount of energy required to break the bonds between the two ions in any compound. Alternately, it can also be defined as the amount of energy released when two ions of opposite charge combine to form a compound of certain lattice structure.
Complete step by step answer:
The lattice structure formation of any chemical compound consists of various different types of energies as the reactants may or may not be present in their ionic state through which they can react easily.
For the formation of sodium chloride crystal lattice structure, it requires five different forms of energies.
The complete cycle that determines the formation of a chemical compound with the help of Lattice energy is known as Born- Haber cycle. The Born- Haber cycle for the formation of $NaCl(s)$is as follows:
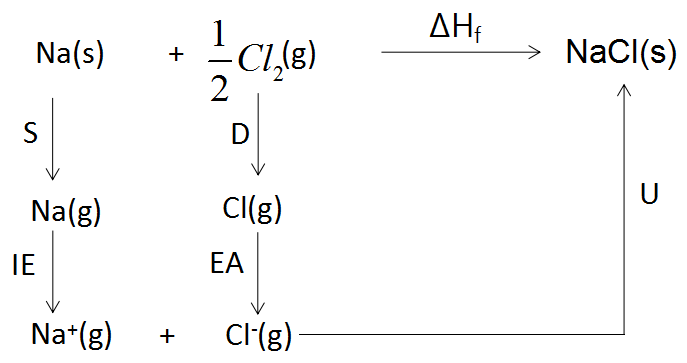
Thus, from the diagram above, we can deduce that the solid sodium is heated at such a high temperature that it sublimes in its gaseous phase and then again it is provided with sufficient ionization enthalpy that helps the sodium gas to release an electron and convert it into its ionic state. Similarly, the chlorine molecule is given sufficient bond dissociation energy that it gets converted into its atomic gaseous state and then it is provided with sufficient electron gain enthalpy such that it gets converted into its corresponding anionic form. Then both the cation and anion react together with the help of sufficient lattice energy to form a solid crystal lattice structure of sodium chloride.
Hence, lattice energy is the sum of all forms of energy along with their signs.
Thus, the correct option is A. Lattice energy$ - U = S + I + \dfrac{D}{2} - E - Q$
Note:
In thermodynamics, when there is a release of energy in any substance, it is denoted with a positive sign and when there is absorption of energy by any substance, it is denoted through a negative sign. The sublimation energy is the amount of heat released and hence, it has a positive sign. Similarly, the ionization enthalpy and bond dissociation enthalpy both have positive signs. The electron gain enthalpy and the heat of formation are both energy gained by the substance and hence, are considered negative.
Complete step by step answer:
The lattice structure formation of any chemical compound consists of various different types of energies as the reactants may or may not be present in their ionic state through which they can react easily.
For the formation of sodium chloride crystal lattice structure, it requires five different forms of energies.
The complete cycle that determines the formation of a chemical compound with the help of Lattice energy is known as Born- Haber cycle. The Born- Haber cycle for the formation of $NaCl(s)$is as follows:
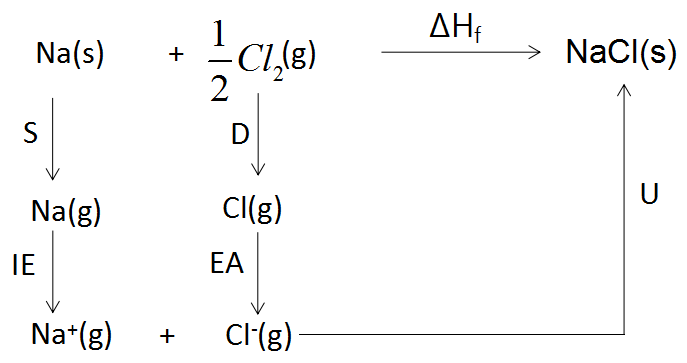
Thus, from the diagram above, we can deduce that the solid sodium is heated at such a high temperature that it sublimes in its gaseous phase and then again it is provided with sufficient ionization enthalpy that helps the sodium gas to release an electron and convert it into its ionic state. Similarly, the chlorine molecule is given sufficient bond dissociation energy that it gets converted into its atomic gaseous state and then it is provided with sufficient electron gain enthalpy such that it gets converted into its corresponding anionic form. Then both the cation and anion react together with the help of sufficient lattice energy to form a solid crystal lattice structure of sodium chloride.
Hence, lattice energy is the sum of all forms of energy along with their signs.
Thus, the correct option is A. Lattice energy$ - U = S + I + \dfrac{D}{2} - E - Q$
Note:
In thermodynamics, when there is a release of energy in any substance, it is denoted with a positive sign and when there is absorption of energy by any substance, it is denoted through a negative sign. The sublimation energy is the amount of heat released and hence, it has a positive sign. Similarly, the ionization enthalpy and bond dissociation enthalpy both have positive signs. The electron gain enthalpy and the heat of formation are both energy gained by the substance and hence, are considered negative.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




