
The keto-enol tautomerism of dicarbonyl compounds; the enol-form is preferred in contrast to the keto form, this is due to:
A. Presence of carbonyl group on each side of $-CH_{2}-$ group
B. Resonance stabilization of enol form
C. Presence of methylene group
D. Rapid chemical exchange
Answer
594.6k+ views
Hint: In a dicarbonyl compound, the enol form is more stable than the keto form because of the delocalization of electrons between the oxygen atom of the carbonyl group and the double bond formed between the two carbon atoms.
Complete answer:
Whenever there is a continuous delocalization of pi-electrons between two alternate atoms or one atom and one pair of bonding electrons, there is a loss of energy from the compound. The more the loss of energy, more the stability the compound achieves. In the case of a dicarbonyl system, the keto form is less stable than the enol form because the enol form is stabilized by the resonance of pi-electrons. This can be explained from the following diagram:
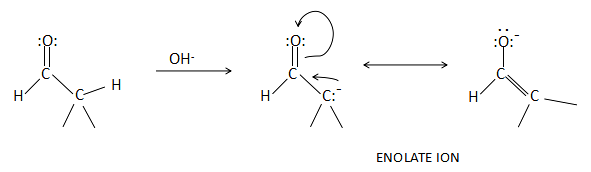
As we can observe from the above pictorial representation, base extracts one hydrogen atom from the dicarbonyl compound to produce a negative charge on the second carbon. This negative charge is delocalized with the pi-electrons of the oxygen atom and loses energy while resonating. The second and the third carbanions are tautomers of each other and the enol form (third structure) is more stable than the keto form. The negative charge is stabilized over more electronegative oxygen atom in the case of enol form. Thus, we can consider it as more stable.
So, the correct answer is Option B .
Note:
Tautomers are the structural isomers of chemical compounds that readily interconvert into their keto and enol form through resonance. This reaction commonly results in the relocation of a proton or delocalization of an electron. The most common example of tautomerism is the zwitterion formation in the amino acids.
Complete answer:
Whenever there is a continuous delocalization of pi-electrons between two alternate atoms or one atom and one pair of bonding electrons, there is a loss of energy from the compound. The more the loss of energy, more the stability the compound achieves. In the case of a dicarbonyl system, the keto form is less stable than the enol form because the enol form is stabilized by the resonance of pi-electrons. This can be explained from the following diagram:

As we can observe from the above pictorial representation, base extracts one hydrogen atom from the dicarbonyl compound to produce a negative charge on the second carbon. This negative charge is delocalized with the pi-electrons of the oxygen atom and loses energy while resonating. The second and the third carbanions are tautomers of each other and the enol form (third structure) is more stable than the keto form. The negative charge is stabilized over more electronegative oxygen atom in the case of enol form. Thus, we can consider it as more stable.
So, the correct answer is Option B .
Note:
Tautomers are the structural isomers of chemical compounds that readily interconvert into their keto and enol form through resonance. This reaction commonly results in the relocation of a proton or delocalization of an electron. The most common example of tautomerism is the zwitterion formation in the amino acids.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




