
The IUPAC name of the given compound is:
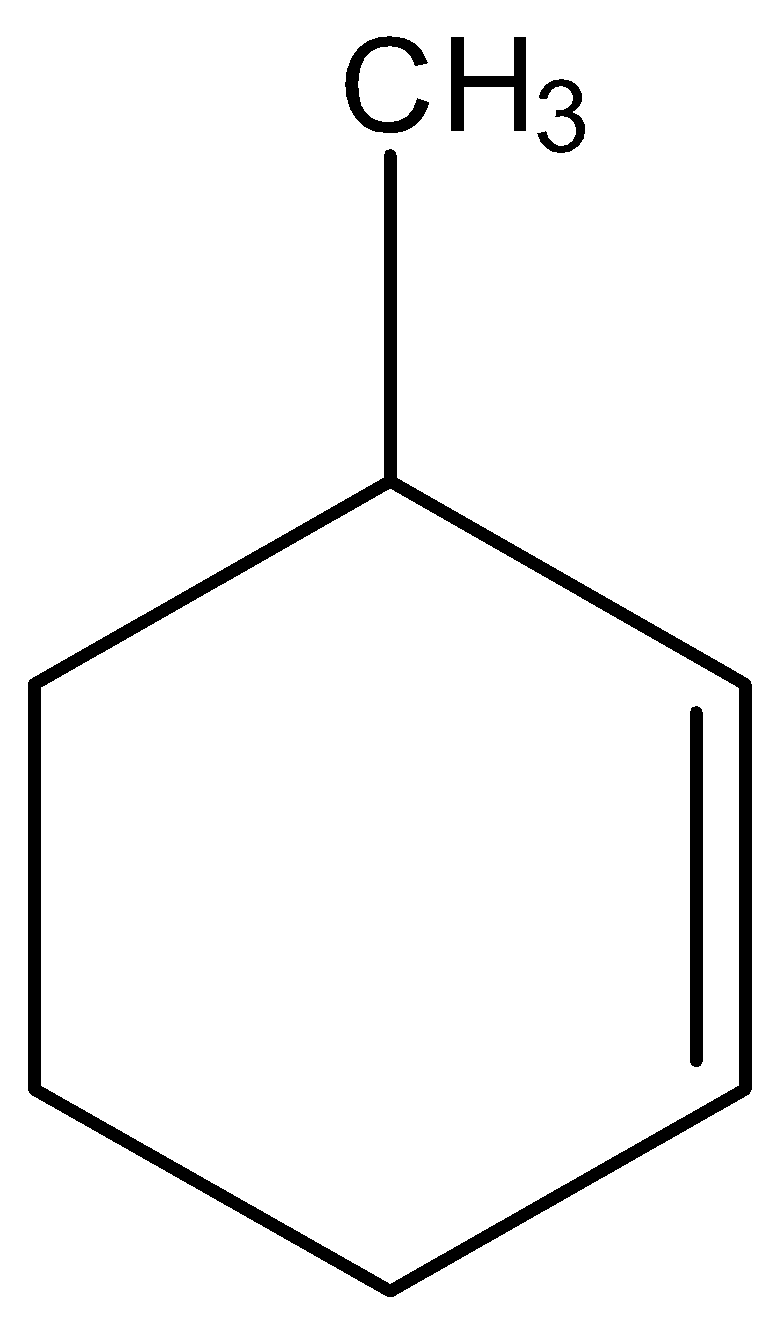
(A) 3-methyl cyclohexene
(B) 1-methyl cyclohex-2-ene
(C) 6-methyl cyclohexene
(D) 1-methyl cyclohex-5-ene

Answer
582k+ views
Hint: If a carbon-carbon double bond is present in the compound, then the functional group is called alkene and we use –ene suffix to show its presence. The alkene carbons should be given lower numbers in the process of numbering the chain.
Complete step by step solution:
We will try to give IUPAC names to the given compound.
- We can see that a carbon-carbon double bond is present in the compound. So, the alkene functional group is present in the compound. We know that –ene is the suffix used to describe the presence of alkene functional groups.
- We can see that there is a cyclic ring present which is made up of six carbons. So, we can call this ring a cyclohexane ring.
- Methyl group is there as a substituent on the cyclohexane ring.
- So, we will take cyclohexane rings as a parent chain of carbons.
- In numbering of the carbons, we should give numbering in a way that both $s{p^2}$ hybridized carbons get the priority. Then the carbon atom bearing methyl group should be considered to have the lowest number.
- Thus, we can give numbers to the carbon atoms in a given manner.
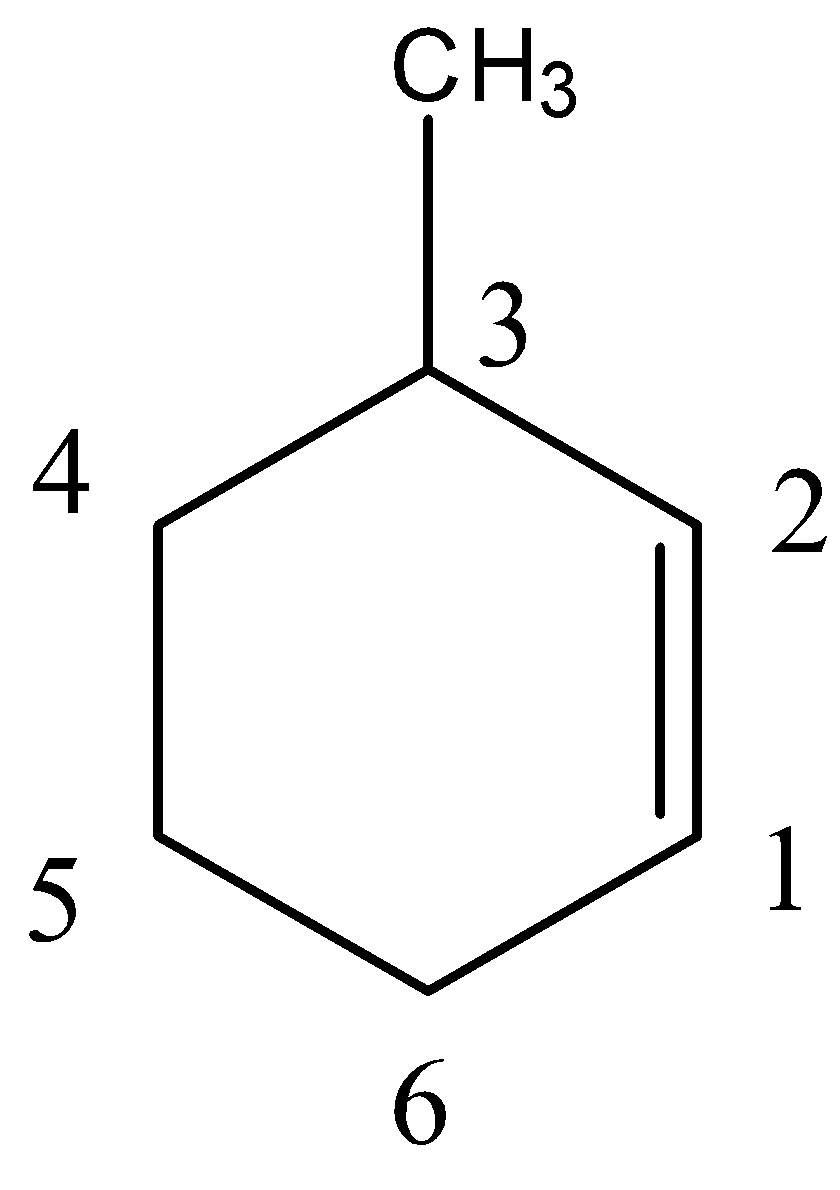
So, we can say that methyl group is present on carbon number 3. We can also say that the alkene functional group is present on carbon number 1. So, the IUPAC name of this compound will be 3-methyl cyclohexene
Therefore, the correct answer of the question is (A).
Note: If more than one similar substituent groups are present, then we can use prefixes like di-, tri-, tetra- etc. If more than one substituent groups are present, then we need to write them in an alphabetical manner in IUPAC name of the compound.
Complete step by step solution:
We will try to give IUPAC names to the given compound.
- We can see that a carbon-carbon double bond is present in the compound. So, the alkene functional group is present in the compound. We know that –ene is the suffix used to describe the presence of alkene functional groups.
- We can see that there is a cyclic ring present which is made up of six carbons. So, we can call this ring a cyclohexane ring.
- Methyl group is there as a substituent on the cyclohexane ring.
- So, we will take cyclohexane rings as a parent chain of carbons.
- In numbering of the carbons, we should give numbering in a way that both $s{p^2}$ hybridized carbons get the priority. Then the carbon atom bearing methyl group should be considered to have the lowest number.
- Thus, we can give numbers to the carbon atoms in a given manner.

So, we can say that methyl group is present on carbon number 3. We can also say that the alkene functional group is present on carbon number 1. So, the IUPAC name of this compound will be 3-methyl cyclohexene
Therefore, the correct answer of the question is (A).
Note: If more than one similar substituent groups are present, then we can use prefixes like di-, tri-, tetra- etc. If more than one substituent groups are present, then we need to write them in an alphabetical manner in IUPAC name of the compound.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




