
The IUPAC name of the following structure is
${[C{H_3}CH(C{H_3})]_2}C(C{H_2}C{H_3})C(C{H_3})C(C{H_2}C{H_3})$
A) 3,5-Diethyl-4,6-dimethyl-5-[1-methylethyl]hept-3-ene
B) 3,5-Diethyl-5-isopropyl-4,6-dimethylhept-2-ene
C) 3,5-Diethyl-5-propyl-4,6-dimethylhept-3-ne
D) None of these
Answer
577.8k+ views
Hint: First you need to draw the branched chemical structure of the given compound. Then, identify the parent chain (the longest carbon chain), all the substituents and functional groups present in the structure. Recall the rules used for naming the branched molecules.
Complete step by step solution:
The given compound is ${[C{H_3}CH(C{H_3})]_2}C(C{H_2}C{H_3})C(C{H_3})C(C{H_2}C{H_3})$.
The chemical structure of given compound can be drawn as:
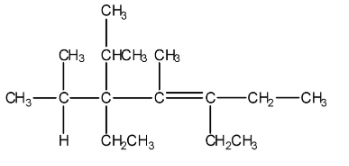
We can see some branched chains in the above structure. The required rules which you must know for naming the naming the branched compounds are:
- First of all the longest carbon chain in the molecule is identified. This is known as the parent chain.
- The numbering of the carbon atoms is done in such a way that the branched carbon atoms get the lowest possible numbers.
- The name of alkyl groups attached as the branch are named as prefixes to the parent chain and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present then, they are listed in alphabetical order.
- If two or more identical substituents are present then the numbers are separated by the commas.
- If a functional group is present in the chain, then it is identified by the appropriate suffix. The parent chain containing the functional group is numbered in such a way that it is attached to the carbon atom possessing lowest possible numbering.
Now, numbering of the above drawn structure can be done as:
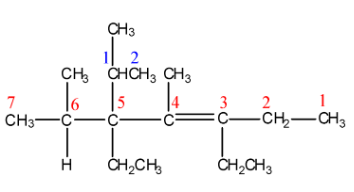
There is a $C = C$ functional group present in the ring, which will be named by suffix ‘ene’. The longest chain containing the functional group has 7 carbon atoms (shown with red-coloured digits); hence, the parent chain is heptane. But, because of the $C = C$ functional group at carbon 3, the parent chain will be named with the suffix ‘3-heptene’. Now, further branching at carbon 5 is numbered with blue-coloured digits. Thus, at carbon 5, numbering of above branching will be done as 5-[1-methylethyl]. At carbon 3 and 5, there is the same substituent present, that is, ethyl group and, at carbon 4 and 6, the same substituent present is methyl group. In summary, we get the IUPAC name of the given structure as: 3,5-Diethyl-4,6-dimethyl-5-[1-methylethyl]hept-3-ene.
Thus, option A is correct.
Note: While writing the IUPAC naming there must be no gap between the prefix and suffix naming. The names of the identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3) etc. are used. Ethyl comes before in alphabetical order than methyl so it is written first before in the naming of given compounds and also a point to notice is that prefix like ‘di’ is not considered while writing naming in alphabetical order.
Complete step by step solution:
The given compound is ${[C{H_3}CH(C{H_3})]_2}C(C{H_2}C{H_3})C(C{H_3})C(C{H_2}C{H_3})$.
The chemical structure of given compound can be drawn as:

We can see some branched chains in the above structure. The required rules which you must know for naming the naming the branched compounds are:
- First of all the longest carbon chain in the molecule is identified. This is known as the parent chain.
- The numbering of the carbon atoms is done in such a way that the branched carbon atoms get the lowest possible numbers.
- The name of alkyl groups attached as the branch are named as prefixes to the parent chain and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present then, they are listed in alphabetical order.
- If two or more identical substituents are present then the numbers are separated by the commas.
- If a functional group is present in the chain, then it is identified by the appropriate suffix. The parent chain containing the functional group is numbered in such a way that it is attached to the carbon atom possessing lowest possible numbering.
Now, numbering of the above drawn structure can be done as:

There is a $C = C$ functional group present in the ring, which will be named by suffix ‘ene’. The longest chain containing the functional group has 7 carbon atoms (shown with red-coloured digits); hence, the parent chain is heptane. But, because of the $C = C$ functional group at carbon 3, the parent chain will be named with the suffix ‘3-heptene’. Now, further branching at carbon 5 is numbered with blue-coloured digits. Thus, at carbon 5, numbering of above branching will be done as 5-[1-methylethyl]. At carbon 3 and 5, there is the same substituent present, that is, ethyl group and, at carbon 4 and 6, the same substituent present is methyl group. In summary, we get the IUPAC name of the given structure as: 3,5-Diethyl-4,6-dimethyl-5-[1-methylethyl]hept-3-ene.
Thus, option A is correct.
Note: While writing the IUPAC naming there must be no gap between the prefix and suffix naming. The names of the identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3) etc. are used. Ethyl comes before in alphabetical order than methyl so it is written first before in the naming of given compounds and also a point to notice is that prefix like ‘di’ is not considered while writing naming in alphabetical order.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




