
The IUPAC name of the \[\text{CC}{{\text{l}}_{\text{4}}}\] is:
A. pyrene
B. Carbon tetrachloride
C. tetrachlorocarbon
D. tetrachloromethane
Answer
604.8k+ views
Hint: We can find the IUPAC name of \[\text{CC}{{\text{l}}_{\text{4}}}\] from the structure, with the help of nomenclature. Once we are able to name the compound from the structure, we can compare it with the options provided.
Complete step-by-step answer:
Below is the structure of \[\text{CC}{{\text{l}}_{\text{4}}}\].
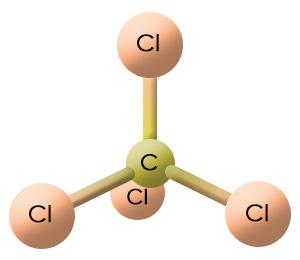
From the structure we can see that carbon is the central atom. There are 4 chlorine atoms as the functional groups of the central atom. So, in order to name the compound, we need to consider the position of the chloride atoms, which are 1,1,1,1.
So the IUPAC name of the compound is 1,1,1,1 - tetracholomethane. Here the name is signed as per the position. Here,
Tetra signifies the secondary prefix for four carbon atoms.
Chloro signifies the primary prefix for chlorine.
Meth signifies the root word for carbon number.
Ane signifies the primary suffix for alkane
Therefore, the name is 1,1,1,1 - tetrachloromethane, which can be written as tetrachloromethane. So the correct option is Option D.
Additional Information: We should have a basic idea about the \[\text{CC}{{\text{l}}_{\text{4}}}\] compound. Here are some properties of \[\text{CC}{{\text{l}}_{\text{4}}}\]:
It is a colourless liquid, which has a sweet smell.
The smell can even be detected at low levels.
Most commonly \[\text{CC}{{\text{l}}_{\text{4}}}\] is known as Pyrene. It is the industrial name of this compound.
It is used for dry cleaning, degreasing metals, fumigating, and in the manufacture of refrigerants and aerosol propellants.
\[\text{CC}{{\text{l}}_{\text{4}}}\] is also used in fire extinguishers because it is heavy non-combustible liquid.
Note: We should know that most commonly \[\text{CC}{{\text{l}}_{\text{4}}}\] is known as Pyrene. It is the industrial name of this compound. It has practically no flammability at lower temperatures.
Complete step-by-step answer:
Below is the structure of \[\text{CC}{{\text{l}}_{\text{4}}}\].

From the structure we can see that carbon is the central atom. There are 4 chlorine atoms as the functional groups of the central atom. So, in order to name the compound, we need to consider the position of the chloride atoms, which are 1,1,1,1.
So the IUPAC name of the compound is 1,1,1,1 - tetracholomethane. Here the name is signed as per the position. Here,
Tetra signifies the secondary prefix for four carbon atoms.
Chloro signifies the primary prefix for chlorine.
Meth signifies the root word for carbon number.
Ane signifies the primary suffix for alkane
Therefore, the name is 1,1,1,1 - tetrachloromethane, which can be written as tetrachloromethane. So the correct option is Option D.
Additional Information: We should have a basic idea about the \[\text{CC}{{\text{l}}_{\text{4}}}\] compound. Here are some properties of \[\text{CC}{{\text{l}}_{\text{4}}}\]:
It is a colourless liquid, which has a sweet smell.
The smell can even be detected at low levels.
Most commonly \[\text{CC}{{\text{l}}_{\text{4}}}\] is known as Pyrene. It is the industrial name of this compound.
It is used for dry cleaning, degreasing metals, fumigating, and in the manufacture of refrigerants and aerosol propellants.
\[\text{CC}{{\text{l}}_{\text{4}}}\] is also used in fire extinguishers because it is heavy non-combustible liquid.
Note: We should know that most commonly \[\text{CC}{{\text{l}}_{\text{4}}}\] is known as Pyrene. It is the industrial name of this compound. It has practically no flammability at lower temperatures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




