
The intermetallic compound LiAg crystallizes in a cubic lattice in which lithium and silver atoms have coordination numbers of 8. To what crystal class does the unit cell belong?
A. Simple cubic
B. Face-centred cubic
C. Body-centred cubic
D. Edge-centred cubic
Answer
570.3k+ views
Hint Two different metals react each other and form a compound which contains both the metals called intermetallic compounds. The properties of the intermetallic compounds resemble the properties of the individual metal atoms.
Complete step by step answer:
- In the question it is given that LiAg crystallizes in a cubic lattice.
- In LiAg crystal lithium and silver have a coordination of 8. We have to find under which crystal structure it comes among the given options.
- In the question itself it is given that the coordination number of lithium and silver atoms is 8.
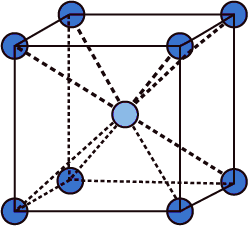
- From the above structure only we can say that each atom will have the coordination of eight.
- The central atom is going to coordinate with eight atoms which are present at the corners of the cube.
- Therefore LiAg crystal belongs to the BCC (body - centered Cubic lattice) unit cell.
- So, the correct option is C.
Note: Sodium chloride crystal belongs to the category face-centered cubic lattice structure due to the presence of sodium atoms at the middle of the faces in the cube. While coming to bcc (body - centered Cubic lattice) the metal atom is going to present in the middle of the cube with a coordination of eight.
Complete step by step answer:
- In the question it is given that LiAg crystallizes in a cubic lattice.
- In LiAg crystal lithium and silver have a coordination of 8. We have to find under which crystal structure it comes among the given options.
- In the question itself it is given that the coordination number of lithium and silver atoms is 8.

- From the above structure only we can say that each atom will have the coordination of eight.
- The central atom is going to coordinate with eight atoms which are present at the corners of the cube.
- Therefore LiAg crystal belongs to the BCC (body - centered Cubic lattice) unit cell.
- So, the correct option is C.
Note: Sodium chloride crystal belongs to the category face-centered cubic lattice structure due to the presence of sodium atoms at the middle of the faces in the cube. While coming to bcc (body - centered Cubic lattice) the metal atom is going to present in the middle of the cube with a coordination of eight.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




