
The hybridization of $ Ni $ in $ Ni{(CO)_4} $ is:
A. $ s{p^2} $
B. $ ds{p^2} $
C. $ s{p^3} $
D. $ s{p^3}d $
Answer
546.6k+ views
Hint :It is a coordination number $ 4 $ complex and the complexes having coordination number $ 4 $ have two geometry that is tetrahedral and square planar. Tetrahedral geometry is shown when the hybridization is $ s{p^3},{d^3}s $ and square planar geometry is shown when the hybridization is $ ds{p^2},s{p^2}d $ .
Complete Step By Step Answer:
Coordination number is the total number of atoms or molecules bonded to the central atom. Coordination number is also called ligancy which means number of ligands bonded to the central metal atom.
In $ Ni{(CO)_4} $ , $ Ni $ is attached to $ 4 $ molecules of $ CO $ that’s why its coordination number is $ 4 $ .
In coordination chemistry, hybridization depends on the number of d-electrons (of the central metal atom). In case of $ Ni{(CO)_4} $ the central metal atom is nickel so we have to count the number of d-electrons in nickel:
Configuration of nickel is as follows:
$ Ni $ = $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2} $
Valence shell configuration: $ 3{d^8}4{s^2} $

As nickel has zero charge on because carbonyl is a neutral ligand now, as we can see that $ s $ is not vacant so $ 4 $ ligands can't get bonded to nickel.
For bonding in such cases, electrons of $ s $ orbital get shifted to $ d $ orbital and new configuration becomes $ 3{d^{10}}4{s^0} $ .
It is called $ d - s $ pairing and the $ {d^{10}} $ is called a converted $ d'system $ .
$ 3{d^{10}}4{s^0}4{p^0} $ :
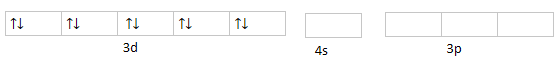 Hybridization will take place with $ s $ and $ p $ orbital as $ s{p^3} $ . Hence, nickel is $ s{p^3} $ hybridized with geometry tetrahedral and spin only moment zero. Spin only moment is zero because the number of unpaired electrons are zero. As one of its properties is also derived by the number of unpaired electrons that is magnetism. There are no unpaired electrons so it is a diamagnetic compound.
Hybridization will take place with $ s $ and $ p $ orbital as $ s{p^3} $ . Hence, nickel is $ s{p^3} $ hybridized with geometry tetrahedral and spin only moment zero. Spin only moment is zero because the number of unpaired electrons are zero. As one of its properties is also derived by the number of unpaired electrons that is magnetism. There are no unpaired electrons so it is a diamagnetic compound.
So, option C is correct.
Note :
$ Ni{(CO)_4} $ was first synthesised in $ 1890 $ by Ludwig Mond by the direct reaction of nickel metal with $ CO $ . This pioneering work foreshadowed the existence of many other metal carbonyl compounds, including those of $ V,Cr,Mn,Fe $ and $ Co $ . It was also applied industrially to the purification of nickel by the end of the nineteenth century.
Complete Step By Step Answer:
Coordination number is the total number of atoms or molecules bonded to the central atom. Coordination number is also called ligancy which means number of ligands bonded to the central metal atom.
In $ Ni{(CO)_4} $ , $ Ni $ is attached to $ 4 $ molecules of $ CO $ that’s why its coordination number is $ 4 $ .
In coordination chemistry, hybridization depends on the number of d-electrons (of the central metal atom). In case of $ Ni{(CO)_4} $ the central metal atom is nickel so we have to count the number of d-electrons in nickel:
Configuration of nickel is as follows:
$ Ni $ = $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2} $
Valence shell configuration: $ 3{d^8}4{s^2} $

As nickel has zero charge on because carbonyl is a neutral ligand now, as we can see that $ s $ is not vacant so $ 4 $ ligands can't get bonded to nickel.
For bonding in such cases, electrons of $ s $ orbital get shifted to $ d $ orbital and new configuration becomes $ 3{d^{10}}4{s^0} $ .
It is called $ d - s $ pairing and the $ {d^{10}} $ is called a converted $ d'system $ .
$ 3{d^{10}}4{s^0}4{p^0} $ :

So, option C is correct.
Note :
$ Ni{(CO)_4} $ was first synthesised in $ 1890 $ by Ludwig Mond by the direct reaction of nickel metal with $ CO $ . This pioneering work foreshadowed the existence of many other metal carbonyl compounds, including those of $ V,Cr,Mn,Fe $ and $ Co $ . It was also applied industrially to the purification of nickel by the end of the nineteenth century.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




