
The hybridization involved in complex ${[Ni{(CN)_4}]^{2 - }}$ is:
[Atomic number of Ni=28]
(A) ${d^2}s{p^2}$
(B) ${d^2}s{p^3}$
(C) $ds{p^2}$
(D) $s{p^3}$
Answer
579.9k+ views
Hint: Hybridization is decided by the orbitals in which the metal ions receive the electrons from ligands via the formation of covalent coordinate bonding. In the process of hybridization, the electronic configuration of metal atoms changes as it attains excited state.
Complete step by step solution:
We are given that the atomic number of Ni is 28. So, we can say that there will be 28 electrons present in the Ni atom.
- We can see that the charge on the complex ${[Ni{(CN)_4}]^{2 - }}$ is (-2). Each cyanide ions has a charge of (-1). So, we can say that Ni atoms are in $ + 2$ oxidation state. Now, we will first write the ground state electronic configuration of $N{i^{2 + }}$ .
Ground state electronic configuration of $N{i^{2 + }}$: $[Ar]3{d^8}$
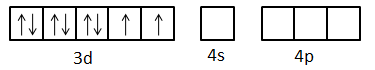
Now, excited state electronic configuration of $N{i^{2 + }}$ can be given as $[Ar]3{d^8}$ but the arrangement of electrons is as below.
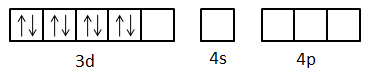
Here, we can see that one d-orbital, one s-orbital and three p-orbitals are empty. The eight electrons present in the d-orbitals are coupled.
- So, the cyanide ligands give the pair of electrons to the empty orbitals of the nickel atom.
- We can see that there are four cyanide ligands present in the complex. So, all four will give one pair of electrons each. So, a total of four orbitals will receive the electron pairs.
- First the orbital with lowest energy receives the electrons. So, electron pairs are received in one d-orbital, one s-orbital and two p-orbitals and in this way, four cyanide ligands bind to the metal.
- So, we can say that the hybridization of the complex is $ds{p^2}$.
So, the correct answer is (C).
Note: As there is one d-orbital, one s-orbital and two p-orbitals are involved in the hybridization, we should write its hybridization in a way that the orbital that has the lowest energy comes first. So, here we have written $ds{p^2}$. We cannot write it as $s{p^2}d$ or $d{p^2}s$.
Complete step by step solution:
We are given that the atomic number of Ni is 28. So, we can say that there will be 28 electrons present in the Ni atom.
- We can see that the charge on the complex ${[Ni{(CN)_4}]^{2 - }}$ is (-2). Each cyanide ions has a charge of (-1). So, we can say that Ni atoms are in $ + 2$ oxidation state. Now, we will first write the ground state electronic configuration of $N{i^{2 + }}$ .
Ground state electronic configuration of $N{i^{2 + }}$: $[Ar]3{d^8}$

Now, excited state electronic configuration of $N{i^{2 + }}$ can be given as $[Ar]3{d^8}$ but the arrangement of electrons is as below.

Here, we can see that one d-orbital, one s-orbital and three p-orbitals are empty. The eight electrons present in the d-orbitals are coupled.
- So, the cyanide ligands give the pair of electrons to the empty orbitals of the nickel atom.
- We can see that there are four cyanide ligands present in the complex. So, all four will give one pair of electrons each. So, a total of four orbitals will receive the electron pairs.
- First the orbital with lowest energy receives the electrons. So, electron pairs are received in one d-orbital, one s-orbital and two p-orbitals and in this way, four cyanide ligands bind to the metal.
- So, we can say that the hybridization of the complex is $ds{p^2}$.
So, the correct answer is (C).
Note: As there is one d-orbital, one s-orbital and two p-orbitals are involved in the hybridization, we should write its hybridization in a way that the orbital that has the lowest energy comes first. So, here we have written $ds{p^2}$. We cannot write it as $s{p^2}d$ or $d{p^2}s$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




