
The geometry of $ Ni{(CO)_4} $ and $ Ni{(PP{h_3})_2}C{l_2} $ are:
(A) Both square planar
(B) Tetrahedral and square planar, respectively
(C) Both tetrahedral
(D) Square planar and tetrahedral, respectively
Answer
521.4k+ views
Hint: First find the electronic configuration of $ Ni $ in $ Ni{(CO)_4} $ and check whether it can be excited or not. Due to the presence of a strong ligand, there will be forced pairing of electrons. Hence, again determine the electronic configuration of $ Ni $ and use it to find the hybridisation which will tell the shape of $ Ni{(CO)_4} $ . The same steps will be applied to find the hybridisation and shape of $ Ni{(PP{h_3})_2}C{l_2} $ .
Complete step by step answer:
Here we will use Valence Band Theory (VBT) to determine the geometry of both $ Ni{(CO)_4} $ and $ Ni{(PP{h_3})_2}C{l_2} $ .
First let’s determine the geometry of $ Ni{(CO)_4} $ .
Atomic number of $ Ni = 28 $
Hence the configuration of $ Ni $ is $ 3{d^8}4{s^2} $ .
The configuration can be calculated as follows:
For an atomic number of $ 28 $ , the electronic configuration is:
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^8},4{s^2} $
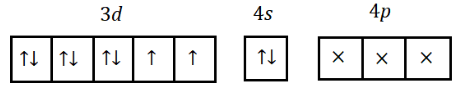
Now if we adjust it for noble-gas configuration, we get,
$ Ni = [Ar]3{d^8}4{s^2} $
We chose Argon because it is the closest element to $ Ni $ and it is a noble-gas. Also, this configuration is in ground state.
But we cannot excite $ Ni $ as the oxidation number of $ Ni $ in $ Ni{(CO)_4} $ is $ 0 $ . However, the electrons from $ s - $ orbital moved to $ d - $ orbital because of the forced pairing of electrons due to the presence of a strong ligand, $ CO $ , and it will change the configuration to:
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^{10}} $
When we draw hybridisation state of $ Ni $ ,
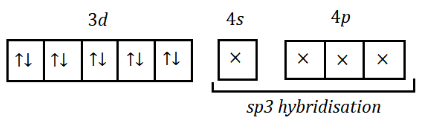
We see that hybridisation is $ s{p^3} $ , and we know that for $ s{p^3} $ hybridisation, the geometrical shape of that molecule is tetrahedral.
Hence, the geometrical shape of $ Ni{(CO)_4} $ will be tetrahedral.
Now, let’s determine the geometry of $ Ni{(PP{h_3})_2}C{l_2} $ .
The configuration of $ Ni $ in ground state will remain same as above,
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^8},4{s^2} $
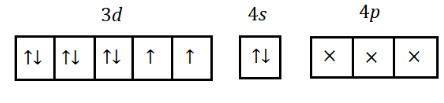
Here, the oxidation number of $ Ni $ in $ Ni{(PP{h_3})_2}C{l_2} $ is $ 2 $ which means $ Ni $ can be excited to $ N{i^{2 + }} $ :
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^{10}} $
There won’t be any forced pairing here because $ C{l_2} $ is a weak ligand.
Again, let’s draw the hybridisation state of $ Ni $ , which is:
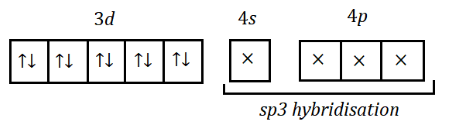
Again, the hybridisation of $ Ni $ is $ s{p^3} $ which results in a tetrahedral geometric shape.
Hence, the geometrical shape of $ Ni{(PP{h_3})_2}C{l_2} $ will be tetrahedral.
Hence option (C) is correct.
Note:
Pay attention while writing the electronic configuration of $ Ni $ in ground state as well as in hybridised state. Make the arrow-head representation to write the electronic configuration as it will help you in verifying the hybridization and electronic configuration. Also, try to remember the shape for each hybridisation since it will save you a lot of time.
Complete step by step answer:
Here we will use Valence Band Theory (VBT) to determine the geometry of both $ Ni{(CO)_4} $ and $ Ni{(PP{h_3})_2}C{l_2} $ .
First let’s determine the geometry of $ Ni{(CO)_4} $ .
Atomic number of $ Ni = 28 $
Hence the configuration of $ Ni $ is $ 3{d^8}4{s^2} $ .
The configuration can be calculated as follows:
For an atomic number of $ 28 $ , the electronic configuration is:
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^8},4{s^2} $

Now if we adjust it for noble-gas configuration, we get,
$ Ni = [Ar]3{d^8}4{s^2} $
We chose Argon because it is the closest element to $ Ni $ and it is a noble-gas. Also, this configuration is in ground state.
But we cannot excite $ Ni $ as the oxidation number of $ Ni $ in $ Ni{(CO)_4} $ is $ 0 $ . However, the electrons from $ s - $ orbital moved to $ d - $ orbital because of the forced pairing of electrons due to the presence of a strong ligand, $ CO $ , and it will change the configuration to:
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^{10}} $
When we draw hybridisation state of $ Ni $ ,

We see that hybridisation is $ s{p^3} $ , and we know that for $ s{p^3} $ hybridisation, the geometrical shape of that molecule is tetrahedral.
Hence, the geometrical shape of $ Ni{(CO)_4} $ will be tetrahedral.
Now, let’s determine the geometry of $ Ni{(PP{h_3})_2}C{l_2} $ .
The configuration of $ Ni $ in ground state will remain same as above,
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^8},4{s^2} $

Here, the oxidation number of $ Ni $ in $ Ni{(PP{h_3})_2}C{l_2} $ is $ 2 $ which means $ Ni $ can be excited to $ N{i^{2 + }} $ :
$ 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^{10}} $
There won’t be any forced pairing here because $ C{l_2} $ is a weak ligand.
Again, let’s draw the hybridisation state of $ Ni $ , which is:

Again, the hybridisation of $ Ni $ is $ s{p^3} $ which results in a tetrahedral geometric shape.
Hence, the geometrical shape of $ Ni{(PP{h_3})_2}C{l_2} $ will be tetrahedral.
Hence option (C) is correct.
Note:
Pay attention while writing the electronic configuration of $ Ni $ in ground state as well as in hybridised state. Make the arrow-head representation to write the electronic configuration as it will help you in verifying the hybridization and electronic configuration. Also, try to remember the shape for each hybridisation since it will save you a lot of time.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




