
The geometry of \[{H_2}S\] and its dipole moment are:
A.Angular and non- zero
B.Angular and zero
C.Linear and non-zero
D.Linear and zero
Answer
585.3k+ views
Hint:We need to find the geometry and hybridization of \[{H_2}S\] molecules. We can find the hybridization by knowing the number of sigma bonds and lone pairs. Geometry of a molecule can be explained by the arrangement of lone pair and bond pairs
Complete step by step answer:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energies, shapes etc, that are suitable for the pairing of electrons to form chemical bonds in valence bond theory.
${\rm{z}}=\;{\rm{no}}{\rm{.}}\;{\rm{of}}\;\sigma\;{\rm{bond}}\;+\;{\rm{l}}{\rm{.p}}\;{\rm{on}}\;{\rm{central}}\;{\rm{atom}}$
Where, No. of $\sigma $bond means single bond and l.p is the lone pair present on the central atom.
For value we have to see the following table.
For \[{H_2}S\],
Z = 2 + 2
Z = 4
\[{H_2}S\] will have $S{p^3}$hybridization and bent molecular geometry and also, \[{H_2}S\] is a nonpolar molecule.
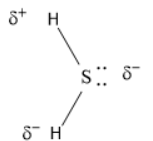
\[{H_2}S\] molecule has an angular geometry because there are two lone pairs of electrons present that make the molecule bend.
As we can see that \[{H_2}S\] is a bent molecule, so the dipole moment is non- zero because sulphur is more electronegative than hydrogen and makes the molecule slightly polar.
The Vectorial sum of the bond dipole moment will produce a non- zero total dipole moment.
Since the permanent dipole moment is non- zero \[{H_2}S\] will show dipole-dipole interactions.
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. It basically occurs when there is a separation of positive and negative charges.
Thus, the geometry of \[{H_2}S\] and its dipole moment are Angular and non- zero.
Note:
Geometry of a molecule can be explained by the arrangement of lone pair and bond pairs while shape can be explained by the arrangement of atoms around the atoms, it is not linked with lone pair and hence only deals with bond pairs.
Complete step by step answer:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energies, shapes etc, that are suitable for the pairing of electrons to form chemical bonds in valence bond theory.
${\rm{z}}=\;{\rm{no}}{\rm{.}}\;{\rm{of}}\;\sigma\;{\rm{bond}}\;+\;{\rm{l}}{\rm{.p}}\;{\rm{on}}\;{\rm{central}}\;{\rm{atom}}$
Where, No. of $\sigma $bond means single bond and l.p is the lone pair present on the central atom.
For value we have to see the following table.
| Z | Hybridisation |
| $2$ | $Sp$ |
| $3$ | $S{p^2}$ |
| $4$ | $S{p^3}$ |
| $5$ | $S{p^3}d$ |

For \[{H_2}S\],
Z = 2 + 2
Z = 4
\[{H_2}S\] will have $S{p^3}$hybridization and bent molecular geometry and also, \[{H_2}S\] is a nonpolar molecule.
\[{H_2}S\] molecule has an angular geometry because there are two lone pairs of electrons present that make the molecule bend.
As we can see that \[{H_2}S\] is a bent molecule, so the dipole moment is non- zero because sulphur is more electronegative than hydrogen and makes the molecule slightly polar.
The Vectorial sum of the bond dipole moment will produce a non- zero total dipole moment.
Since the permanent dipole moment is non- zero \[{H_2}S\] will show dipole-dipole interactions.
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. It basically occurs when there is a separation of positive and negative charges.
Thus, the geometry of \[{H_2}S\] and its dipole moment are Angular and non- zero.
Note:
Geometry of a molecule can be explained by the arrangement of lone pair and bond pairs while shape can be explained by the arrangement of atoms around the atoms, it is not linked with lone pair and hence only deals with bond pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




