
The geometry of $CC{{l}_{4}}$ is:
A. Linear
B. Planar
C. Bent
D. Tetrahedral
Answer
564.9k+ views
Hint: $CC{{l}_{4}}$ is known by the name carbon tetrachloride which is an organic compound. Generally a colorless liquid having a sweet smell. At low temperature it has no flammability therefore it is commonly used in fire extinguishers.
Complete Solution :
To know about the molecular geometry of any compound we firstly have to learn about the hybridization of that compound. Hybridization is defined as the mixing of the atomic orbitals belonging to the same compound but have slightly different energies so that redistribution of energy takes place between them which results in the formation of new orbitals of equal energies and new orbitals formed are known as hybrid orbitals.
- In the compound $CC{{l}_{4}}$ there are four covalent bonds between the central carbon atom and four chlorine atoms and all the valance electrons of carbon takes participate in bond formation and all orbitals of the atom participate in the formation of hybrid orbitals. In $CC{{l}_{4}}$ one s and three p orbitals of carbon atom hybridize and form a $s{{p}^{3}}$ hybridization. In this compound carbon is present in the central position and rest all the chlorine atoms are placed around it. As the central metal atom has four bonded pairs and shows $s{{p}^{3}}$hybridization therefore the shape of the molecule is tetrahedral. Which can be shown as:
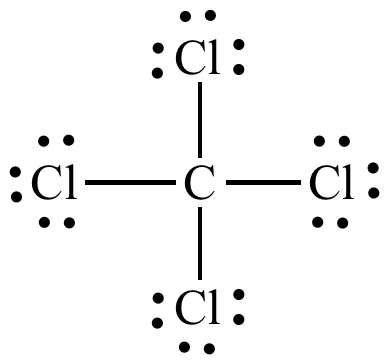
So, the correct answer is “Option D”.
Note: Carbon tetrachloride used as a source of chlorine in the Appel reaction and also used in stamp collecting to reveal watermarks on postage stamps without damaging it and can be used as dry cleaning solvent, refrigerant and in lava lamps.
Complete Solution :
To know about the molecular geometry of any compound we firstly have to learn about the hybridization of that compound. Hybridization is defined as the mixing of the atomic orbitals belonging to the same compound but have slightly different energies so that redistribution of energy takes place between them which results in the formation of new orbitals of equal energies and new orbitals formed are known as hybrid orbitals.
- In the compound $CC{{l}_{4}}$ there are four covalent bonds between the central carbon atom and four chlorine atoms and all the valance electrons of carbon takes participate in bond formation and all orbitals of the atom participate in the formation of hybrid orbitals. In $CC{{l}_{4}}$ one s and three p orbitals of carbon atom hybridize and form a $s{{p}^{3}}$ hybridization. In this compound carbon is present in the central position and rest all the chlorine atoms are placed around it. As the central metal atom has four bonded pairs and shows $s{{p}^{3}}$hybridization therefore the shape of the molecule is tetrahedral. Which can be shown as:

So, the correct answer is “Option D”.
Note: Carbon tetrachloride used as a source of chlorine in the Appel reaction and also used in stamp collecting to reveal watermarks on postage stamps without damaging it and can be used as dry cleaning solvent, refrigerant and in lava lamps.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




