
The general formula ${C_n}{H_{2n}}$is valid for alkene with ___.
A. $2$ double bond
B. $1$ double bond
C. $3$ double bond
D. None of these
Answer
569.7k+ views
Hint: We have to remember that alkenes are acyclic (branched hydrocarbons or unbranched hydrocarbons), characterized by the presence of a carbon-to-carbon double bond. The general molecular formula for alkene is ${C_n}{H_{2n}}$. Alkenes contain two hydrogen atoms less than the parent alkanes (saturated aliphatic hydrocarbons), so they are said to be unsaturated.
Complete step by step answer:
In the chemistry of compounds, alkenes are big different to other compounds, because of the one double bond.
The simple hydrocarbons alkanes, alkenes and alkynes should not be confused. Alkanes are the simplest organic compounds and have only single bonds and also called saturated hydrocarbons.
We have to remember that alkenes are unsaturated hydrocarbons with one carbon-carbon double bond. Alkynes are linear unsaturated acyclic hydrocarbons and contain carbon-carbon triple bonds. Alkyne is also called unsaturated hydrocarbons.
The general molecular formula for alkanes, alkenes and alkynes are ${C_n}{H_{2n}}_{ + 2}$,${C_n}{H_{2n}}$ and ${C_n}{H_{2n - 2}}$.
We have to remember that alkenes are open chain hydrocarbons and are also called olefins, some of earliest products derived from alkenes were oily (from crude oil) in appearance. So called olefins. Due to the presence of double bond alkenes are more reactive than the alkanes.
The general molecular formula ${C_n}{H_{2n}}$is for the homologous series of alkenes, having one carbon-to-carbon double bond $(C = C)$.
For example, the first few alkenes are given below,
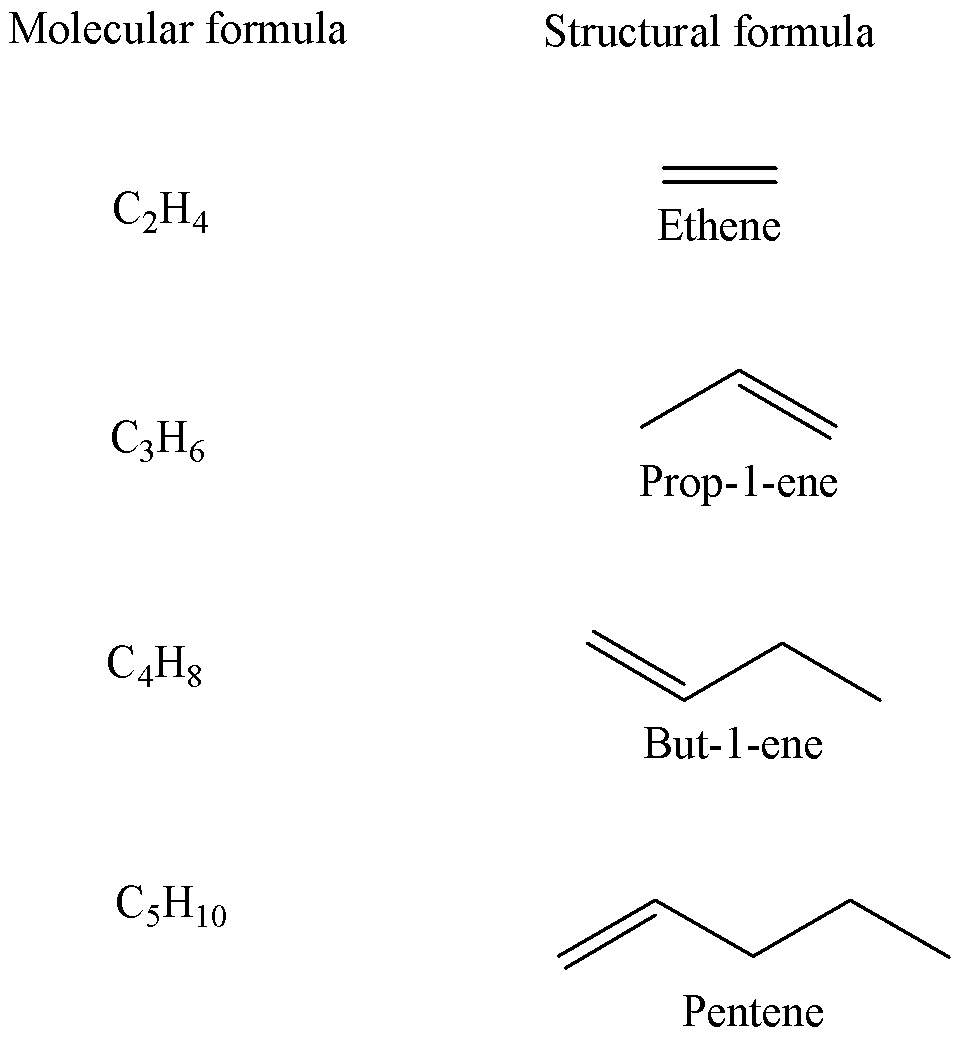
So, the correct answer is Option B.
Note: We have to remember that the alkenes have major commercial uses of production of polymers and important starting material in many consumer chemicals used in a variety of many consumer products. And also a major place in the biological world and also perfumes, flavors and fragments.
Complete step by step answer:
In the chemistry of compounds, alkenes are big different to other compounds, because of the one double bond.
The simple hydrocarbons alkanes, alkenes and alkynes should not be confused. Alkanes are the simplest organic compounds and have only single bonds and also called saturated hydrocarbons.
We have to remember that alkenes are unsaturated hydrocarbons with one carbon-carbon double bond. Alkynes are linear unsaturated acyclic hydrocarbons and contain carbon-carbon triple bonds. Alkyne is also called unsaturated hydrocarbons.
The general molecular formula for alkanes, alkenes and alkynes are ${C_n}{H_{2n}}_{ + 2}$,${C_n}{H_{2n}}$ and ${C_n}{H_{2n - 2}}$.
We have to remember that alkenes are open chain hydrocarbons and are also called olefins, some of earliest products derived from alkenes were oily (from crude oil) in appearance. So called olefins. Due to the presence of double bond alkenes are more reactive than the alkanes.
The general molecular formula ${C_n}{H_{2n}}$is for the homologous series of alkenes, having one carbon-to-carbon double bond $(C = C)$.
For example, the first few alkenes are given below,

So, the correct answer is Option B.
Note: We have to remember that the alkenes have major commercial uses of production of polymers and important starting material in many consumer chemicals used in a variety of many consumer products. And also a major place in the biological world and also perfumes, flavors and fragments.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




