
The functional group present in organic acid is-
A)-OH B)-CHO C)-COOH D)>C=O
Answer
579.6k+ views
Hint: According to the definition of acid, the molecules which dissociate in water and give H+ easily are acids. So the functional group present in the organic acid will have the same property.
Complete step by step answer:
The functional group present in organic acid is –COOH (carboxyl group) which consists of a carbonyl group(C=O) with a hydroxyl group (-OH).It dissociates in water as COO- and H+. The reaction is as follows-
$ \Rightarrow {\text{RCOOH}} \rightleftharpoons {\text{RCO}}{{\text{O}}^ - } + {{\text{H}}^ + }$
Due to this functional group, the organic acids get their properties.
The physical properties of carboxylic acids are-
The boiling point of these acids is higher than that of alcohols, ketones or aldehydes having similar molecular weight.
The lower weight carboxylic acids (containing four carbon atoms) are miscible in water as they form hydrogen bonds in water.
Hence the correct answer is ‘C’.
Additional information:
The organic acids are known as carboxylic acid as the functional group is carboxyl group.
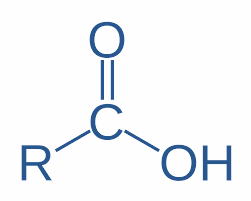
This is the structure of the organic acid where R can be an alkyl or benzyl group and –COOH is the functional group. They are known as carboxylic acids. Their general formula is ${{\text{C}}_n}{{\text{H}}_{2n}}{{\text{O}}_2}$. The –CHO ,>C=O and –OH group are the functional groups of aldehyde, ketone and alcohol respectively and they do not give H+ in water as easily as carboxyl groups can do.
The important properties of these acids are-
-They turn blue litmus to red.
-They react with bases to form salt of carboxylic acids.
$ \Rightarrow {\text{RCOOH + NaOH}} \to {\text{RCOONa + }}{{\text{H}}_2}{\text{O}}$
Note:
The uses of carboxylic acids are-
-Methanoic acid is used in rubber, textile, dyeing, leather and the electroplating industry.
-Ethanoic acid is used as solvent and its diluted form is used as vinegar.
-It is used in manufacture of ${\text{nylon - 6,6}}$
-The higher fatty acids are used in the manufacture of soaps and detergents.
Complete step by step answer:
The functional group present in organic acid is –COOH (carboxyl group) which consists of a carbonyl group(C=O) with a hydroxyl group (-OH).It dissociates in water as COO- and H+. The reaction is as follows-
$ \Rightarrow {\text{RCOOH}} \rightleftharpoons {\text{RCO}}{{\text{O}}^ - } + {{\text{H}}^ + }$
Due to this functional group, the organic acids get their properties.
The physical properties of carboxylic acids are-
The boiling point of these acids is higher than that of alcohols, ketones or aldehydes having similar molecular weight.
The lower weight carboxylic acids (containing four carbon atoms) are miscible in water as they form hydrogen bonds in water.
Hence the correct answer is ‘C’.
Additional information:
The organic acids are known as carboxylic acid as the functional group is carboxyl group.

This is the structure of the organic acid where R can be an alkyl or benzyl group and –COOH is the functional group. They are known as carboxylic acids. Their general formula is ${{\text{C}}_n}{{\text{H}}_{2n}}{{\text{O}}_2}$. The –CHO ,>C=O and –OH group are the functional groups of aldehyde, ketone and alcohol respectively and they do not give H+ in water as easily as carboxyl groups can do.
The important properties of these acids are-
-They turn blue litmus to red.
-They react with bases to form salt of carboxylic acids.
$ \Rightarrow {\text{RCOOH + NaOH}} \to {\text{RCOONa + }}{{\text{H}}_2}{\text{O}}$
Note:
The uses of carboxylic acids are-
-Methanoic acid is used in rubber, textile, dyeing, leather and the electroplating industry.
-Ethanoic acid is used as solvent and its diluted form is used as vinegar.
-It is used in manufacture of ${\text{nylon - 6,6}}$
-The higher fatty acids are used in the manufacture of soaps and detergents.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




