
The four sigma bonds in perchlorate ion are as follows:
A.$s{{p}^{3}}-s{{p}^{3}}$ bond
B.$s{{p}^{3}}-p$ bond
C.$s{{p}^{2}}-s{{p}^{2}}$ bond
D.$s{{p}^{2}}-p$ bond
Answer
585k+ views
Hint: We know Hybridization happens when the atom bonds, using electrons from both the S and P -orbitals, create a difference in the energy levels of the electrons. In order to equalise these energy levels, the corresponding s and p orbitals are combined to create hybrid orbitals.
Complete step by step answer:
As we know sigma and pi bonds include the process of hybridisation.
Talking about the concepts of hybridization it refers to the mixing of atomic orbitals into new hybrid orbitals (with different energies, shapes, etc.,) suitable for the pairing of electrons to make chemical bonds in valence bond theory. Hybrid orbitals are very useful within the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space.
About Perchlorate, it is defined as a monovalent inorganic anion obtained by deprotonation of perchloric acid. It's a monovalent inorganic anion and a chlorine oxo anion. it's a conjugate base of a perchloric acid.
As the question is related to sigma and pi bond here is its general definition:
The covalent bond formed by the coaxial overlap of atomic orbitals is termed as sigma bonding, for example methane molecules contain $4\text{ }C-H$ sigma bonding.
The covalent bond formed by lateral overlap of atomic orbitals is termed as pi bond. As an example, ethylene molecules contain 5 sigma bonding and 1 pi bonding in it.
According the question the formation of perchlorate ion is below
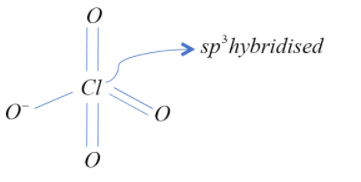
Therefore, all four bonds are $s{{p}^{3}}-p$ bond as oxygen is meant to bring its $p-$orbital for bonding.
Hence Option B is the correct answer of the question.
Note:
Here are some Hybridization points which involves combining and recasting
the same element of atomic orbitals.
The orbitals involved in this process must have almost the same energy.
Only atomic orbitals, not electrons, undergo hybridization.
Number of hybrid orbitals produced = number of hybrid orbitals involved in hybridisation.
Complete step by step answer:
As we know sigma and pi bonds include the process of hybridisation.
Talking about the concepts of hybridization it refers to the mixing of atomic orbitals into new hybrid orbitals (with different energies, shapes, etc.,) suitable for the pairing of electrons to make chemical bonds in valence bond theory. Hybrid orbitals are very useful within the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space.
About Perchlorate, it is defined as a monovalent inorganic anion obtained by deprotonation of perchloric acid. It's a monovalent inorganic anion and a chlorine oxo anion. it's a conjugate base of a perchloric acid.
As the question is related to sigma and pi bond here is its general definition:
The covalent bond formed by the coaxial overlap of atomic orbitals is termed as sigma bonding, for example methane molecules contain $4\text{ }C-H$ sigma bonding.
The covalent bond formed by lateral overlap of atomic orbitals is termed as pi bond. As an example, ethylene molecules contain 5 sigma bonding and 1 pi bonding in it.
According the question the formation of perchlorate ion is below

Therefore, all four bonds are $s{{p}^{3}}-p$ bond as oxygen is meant to bring its $p-$orbital for bonding.
Hence Option B is the correct answer of the question.
Note:
Here are some Hybridization points which involves combining and recasting
the same element of atomic orbitals.
The orbitals involved in this process must have almost the same energy.
Only atomic orbitals, not electrons, undergo hybridization.
Number of hybrid orbitals produced = number of hybrid orbitals involved in hybridisation.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




