
The formula of Marshall’s acid is :
(A) $ {H_2}S{O_5} $
(B) $ {H_2}{S_2}{O_7} $
Answer
544.5k+ views
Hint: Here the question is of the Marshall’s acid and the acid is the inorganic acid of sulphur. The given acid is prepared by the obtained reaction of chloro sulphuric acid with the compound of hydrogen peroxide. Therefore, we get that the chemical formula is this $ H{O_3}SOOS{O_3}H $ .
Complete step by step solution:
The given question is regarding the compound which is known as Marshall's acid. This is an inorganic compound with sulphur, oxygen and hydrogen forming most of it.
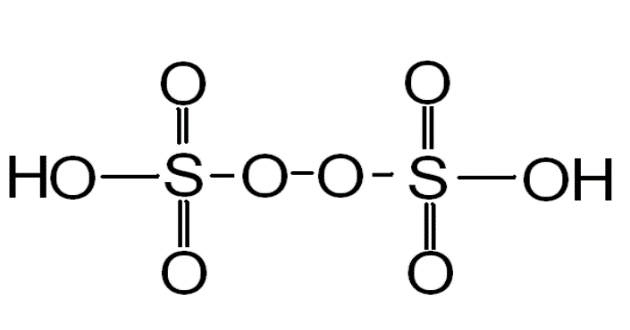
The Marshall's acid is an oxy acid of Sulphur and it has a peroxide linkage in them i.e. they have (–O-O-) linkage in them. The Marshall acid is also called as Peroxodisulphuric acid.
It is called Marshall's acid after its inventor Professor Hugh Marshall, it is a type of sulphur oxoacid. In structural terms it can be written $ H{O_3}SOOS{O_3}H $
SO here we get that the solution of the problem statement is $ H{O_3}SOOS{O_3}H $ .
Therefore, the correct option would be option B. $ {H_2}{S_2}{O_7} $ .
Note:
It contains sulphur in its +6 oxidation state and a peroxide group. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents. In the compound sulphur is $ S{P^3} $ hybridized.
Complete step by step solution:
The given question is regarding the compound which is known as Marshall's acid. This is an inorganic compound with sulphur, oxygen and hydrogen forming most of it.

The Marshall's acid is an oxy acid of Sulphur and it has a peroxide linkage in them i.e. they have (–O-O-) linkage in them. The Marshall acid is also called as Peroxodisulphuric acid.
It is called Marshall's acid after its inventor Professor Hugh Marshall, it is a type of sulphur oxoacid. In structural terms it can be written $ H{O_3}SOOS{O_3}H $
SO here we get that the solution of the problem statement is $ H{O_3}SOOS{O_3}H $ .
Therefore, the correct option would be option B. $ {H_2}{S_2}{O_7} $ .
Note:
It contains sulphur in its +6 oxidation state and a peroxide group. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents. In the compound sulphur is $ S{P^3} $ hybridized.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




