
What will be the formula and electron dot structure of cyclopentane?
Answer
529.1k+ views
Hint:Cyclopentane is an alicyclic compound with formula ${{\text{C}}_5}{{\text{H}}_{10}}$ . The electron dot structure helps us to visualise the valence electrons and molecules whether they are present in bond or as lone pair.
Complete step by step answer:
As we know cyclopentane is an alicyclic hydrocarbon with chemical formula ${{\text{C}}_5}{{\text{H}}_{10}}$ consisting of a five carbon atom ring each atom bonded with two hydrogen atom. It is a clear and colorless liquid having mild sweet odor. Cyclopentane or any cyclohexane can be formulated via known catalytic reforming. Cyclopentane is a flammable liquid. It was first prepared by German chemist Johannes Wislicenus in $1893$ . Cyclopentane is used in the manufacture of rubber adhesive and synthetic resin. It is also used as a blowing agent in the manufacture of insulating foam which is used in many refrigerating appliances from above, we got to know that chemical formula of cyclopentane is ${{\text{C}}_5}{{\text{H}}_{10}}$ .
Electron dot structure is also known as Lewis dot structure which helps to visualise the valence electrons of atoms and molecules whether they exist as lone pairs or within the bonds. A Lewis structure can be drawn for any covalently bonded molecules. The electron dot structure was first introduced by Gilbert N. Lewis in $1916$. The structure of cyclopentane is as follow:
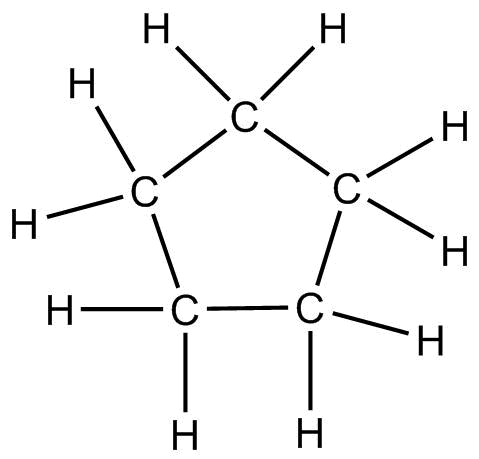
The electron dot representation of cyclopentane is:
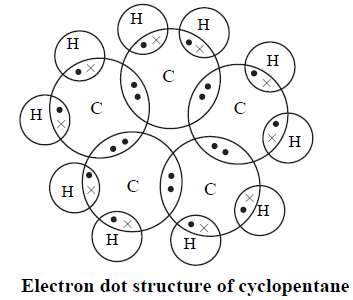
In the above electron dot structure, the dot symbol represents the electrons of carbon atoms whereas the cross $\left( \times \right)$ symbol represents the electron of hydrogen atom. This helps to easily visualise and differentiate between the electrons of the atoms bonded.
Note:
Cyclopentane is a highly flammable alicyclic hydrocarbon which consists of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It has a petrol-like odor and occurs as a colorless liquid.
Complete step by step answer:
As we know cyclopentane is an alicyclic hydrocarbon with chemical formula ${{\text{C}}_5}{{\text{H}}_{10}}$ consisting of a five carbon atom ring each atom bonded with two hydrogen atom. It is a clear and colorless liquid having mild sweet odor. Cyclopentane or any cyclohexane can be formulated via known catalytic reforming. Cyclopentane is a flammable liquid. It was first prepared by German chemist Johannes Wislicenus in $1893$ . Cyclopentane is used in the manufacture of rubber adhesive and synthetic resin. It is also used as a blowing agent in the manufacture of insulating foam which is used in many refrigerating appliances from above, we got to know that chemical formula of cyclopentane is ${{\text{C}}_5}{{\text{H}}_{10}}$ .
Electron dot structure is also known as Lewis dot structure which helps to visualise the valence electrons of atoms and molecules whether they exist as lone pairs or within the bonds. A Lewis structure can be drawn for any covalently bonded molecules. The electron dot structure was first introduced by Gilbert N. Lewis in $1916$. The structure of cyclopentane is as follow:

The electron dot representation of cyclopentane is:

In the above electron dot structure, the dot symbol represents the electrons of carbon atoms whereas the cross $\left( \times \right)$ symbol represents the electron of hydrogen atom. This helps to easily visualise and differentiate between the electrons of the atoms bonded.
Note:
Cyclopentane is a highly flammable alicyclic hydrocarbon which consists of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It has a petrol-like odor and occurs as a colorless liquid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




