
The following transformation involves a carbocation rearrangement. The carbocation is generated by protonation of the hydroxyl group, followed by the loss of water. Which bond has to migrate in the carbocation to yield the product indicated (after the deprotonation)?
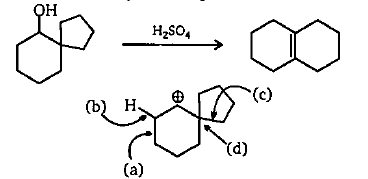
A. a
B. b
C. c
D. d

Answer
589.2k+ views
Hint: We must know that whenever a carbocation is formed near a ring then that ring will have a ring expansion. Also, the 5-membered ring is less stable as compared with 6-membered one.
Complete answer:
Let’s start with discussing how the carbocation is being formed, when the hydrogen ion \[\left( {{H^ + }} \right)\] attacks the hydroxide attached with carbon, the hydroxide gets removed, converted to hydroxide ion \[\left( {O{H^ - }} \right)\] leaving a positive charge on the carbon it is attached with, which leads to the formation of carbocation. The hydroxide ion and hydrogen ion combined to give water.
It is important to know that whenever a carbocation is formed near a ring then that ring will have a ring expansion. For example, the 4-membered ring will become 5-membered or 5-membered will become 6-membered. We also know that a 5-membered ring is less stable whereas the 6-membered ring is highly stable. So, the carbocation formed will not disturb the six membered ring. So, the ring that will be expanded will be the 5-membered ring to 6-membered ring so that it becomes more stable.
This means only one bond out of the four bonds given can be broken i.e.C. and after breaking the positive ion moved to the next carbon a bonding will happen resulting in a six membered ring. The positive ion is still there so hydrogen will be removed from nearby carbon and a double bond will be formed.
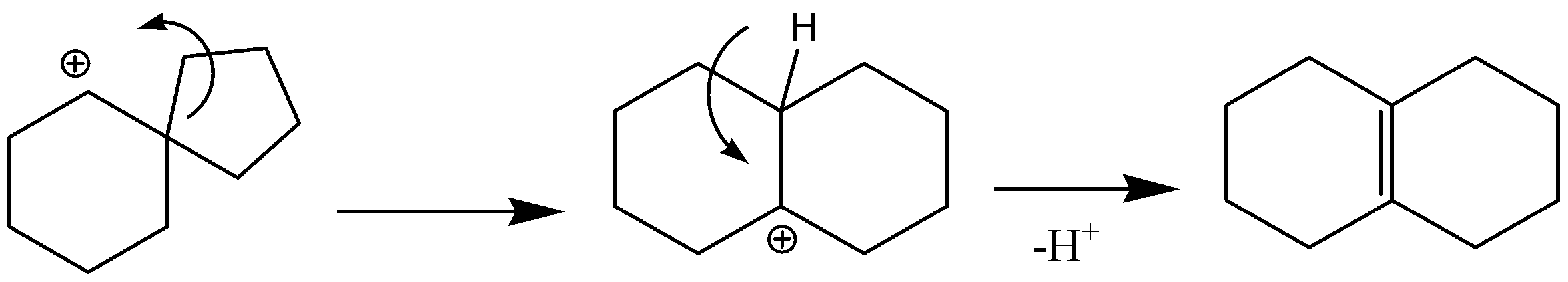
So, the correct answer is “Option C”.
Note: We must know that the stability of a ring is an important factor for any ring based compound as it states its properties and whether it goes under some reactions or not as we have seen above in this question. The six membered ring is more stable as compared with the 5-membered one, hence 5-membered rings always try to become a six membered ring as everything aims for stability.
Complete answer:
Let’s start with discussing how the carbocation is being formed, when the hydrogen ion \[\left( {{H^ + }} \right)\] attacks the hydroxide attached with carbon, the hydroxide gets removed, converted to hydroxide ion \[\left( {O{H^ - }} \right)\] leaving a positive charge on the carbon it is attached with, which leads to the formation of carbocation. The hydroxide ion and hydrogen ion combined to give water.
It is important to know that whenever a carbocation is formed near a ring then that ring will have a ring expansion. For example, the 4-membered ring will become 5-membered or 5-membered will become 6-membered. We also know that a 5-membered ring is less stable whereas the 6-membered ring is highly stable. So, the carbocation formed will not disturb the six membered ring. So, the ring that will be expanded will be the 5-membered ring to 6-membered ring so that it becomes more stable.
This means only one bond out of the four bonds given can be broken i.e.C. and after breaking the positive ion moved to the next carbon a bonding will happen resulting in a six membered ring. The positive ion is still there so hydrogen will be removed from nearby carbon and a double bond will be formed.

So, the correct answer is “Option C”.
Note: We must know that the stability of a ring is an important factor for any ring based compound as it states its properties and whether it goes under some reactions or not as we have seen above in this question. The six membered ring is more stable as compared with the 5-membered one, hence 5-membered rings always try to become a six membered ring as everything aims for stability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




