
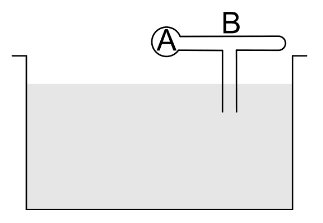
The figure shows a model of perfume atomizer. When the bulb A is compressed, air flows through the narrow tube consequently pressure at the position of the vertical tube reduces. The liquid (perfume) rises in through the vertical tube and emerges through the end. If the excess pressure applied to the bulb in this process be $\Delta P$ then the minimum speed of air in the tube to lift the perfume is
A. $\sqrt {\dfrac{{2{\text{ }}(\Delta P{\text{ }} + {\text{ }}\rho gh)}}{{{\rho _a}}}} \:$
B. $\sqrt {\dfrac{{2{\text{ }}(\Delta P{\text{ }} - {\text{ }}\rho gh)}}{{{\rho _a}}}} \:$
C. $\sqrt {\dfrac{{\Delta P{\text{ }} + {\text{ }}\rho gh}}{{{\rho _a}}}} \:$
D. None of these

Answer
511.2k+ views
Hint: We will use the concept of gauge pressure. Then, we will perform some minute manipulations. Then we will finally find the equation of the required parameter. Finally, we will compare the evaluated equation with the given options and also manipulate if necessary.
Formula Used:
${P_o}{\text{ }} = {\text{ }}P{\text{ }} + {\text{ }}\rho gh$
Where, ${P_o}$ is the atmospheric pressure, $P$ is the pressure on a substance and $\rho gh$ is called the gauge pressure on the substance.
Energy per unit volume: $\dfrac{1}{2}{\text{ }}\rho {V^2}$
Where, $V$ is the volume of the substance.
Complete step by step answer:
The pressure on the bulb B can be written as the pressure on B summed up with the energy per unit volume due to A. Thus,
$P{\text{ }} = {\text{ }}{P_B}{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}{\text{ }} - - - - - - - - - - {\text{ }}(i)$
Again, the pressure on bulb B is the excess pressure summed up with the atmospheric pressure.Thus,
$P{\text{ }} = {\text{ }}\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} - - - - - - - - - - {\text{ }}(ii)$
Using $(ii)$ in $(i)$, we get
$\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} = {\text{ }}{P_B}{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}{\text{ }} - - - - - - - {\text{ }}(iii)$
Now, we can write,
${P_B}{\text{ }} + {\text{ }}\rho gh{\text{ }} = {\text{ }}{P_o}$
Further, we get
${P_B}{\text{ }} = {\text{ }}{P_o}{\text{ }} - {\text{ }}\rho gh{\text{ }} - - - - - - - - - {\text{ }}(iv)$
Using $(iv)$ in $(iii)$, we get
$\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} = {\text{ }}{P_o}{\text{ }} - {\text{ }}\rho gh{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Clearly, ${P_o}$ will cancel out from both sides. Thus, we get
$\Delta P{\text{ }} = {\text{ }} - {\text{ }}\rho gh{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Further, we get
$\Delta P{\text{ }} + {\text{ }}\rho gh{\text{ }} = {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Then, we can write
$2{\text{ }}\left( {\Delta P{\text{ }} + {\text{ }}\rho gh} \right){\text{ }} = {\text{ }}{\rho _a}{V^2}$
Further, we get
$\dfrac{{2{\text{ }}\left( {\Delta P{\text{ }} + {\text{ }}\rho gh} \right)}}{{{\rho _a}}}{\text{ }} = {\text{ }}{V^2}$
Finally, we get
$\therefore V{\text{ }} = {\text{ }}\sqrt {\dfrac{{2{\text{ }}(\Delta P{\text{ }} + {\text{ }}\rho gh)}}{{{\rho _a}}}} $
Hence, the correct answer is B.
Additional information: Gauge pressure is the pressure which is excessive of the atmospheric pressure acting on a substance. Absolute pressure on a substance is simply the summation of the gauge pressure and the atmospheric pressure. This gauge pressure could be of any type. Let us say if an object is immersed in a liquid, then the gauge pressure here will be the pressure the liquid exerts on the walls of the object. Like this, the gauge pressure on an object will be dependent on the situation in which the object is kept in.
Note: Students should be very much cautious while substituting the value of gauge pressure as the gauge pressure is different in different cases. Students commit errors while manipulating the equations.
Formula Used:
${P_o}{\text{ }} = {\text{ }}P{\text{ }} + {\text{ }}\rho gh$
Where, ${P_o}$ is the atmospheric pressure, $P$ is the pressure on a substance and $\rho gh$ is called the gauge pressure on the substance.
Energy per unit volume: $\dfrac{1}{2}{\text{ }}\rho {V^2}$
Where, $V$ is the volume of the substance.
Complete step by step answer:
The pressure on the bulb B can be written as the pressure on B summed up with the energy per unit volume due to A. Thus,
$P{\text{ }} = {\text{ }}{P_B}{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}{\text{ }} - - - - - - - - - - {\text{ }}(i)$
Again, the pressure on bulb B is the excess pressure summed up with the atmospheric pressure.Thus,
$P{\text{ }} = {\text{ }}\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} - - - - - - - - - - {\text{ }}(ii)$
Using $(ii)$ in $(i)$, we get
$\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} = {\text{ }}{P_B}{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}{\text{ }} - - - - - - - {\text{ }}(iii)$
Now, we can write,
${P_B}{\text{ }} + {\text{ }}\rho gh{\text{ }} = {\text{ }}{P_o}$
Further, we get
${P_B}{\text{ }} = {\text{ }}{P_o}{\text{ }} - {\text{ }}\rho gh{\text{ }} - - - - - - - - - {\text{ }}(iv)$
Using $(iv)$ in $(iii)$, we get
$\Delta P{\text{ }} + {\text{ }}{P_o}{\text{ }} = {\text{ }}{P_o}{\text{ }} - {\text{ }}\rho gh{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Clearly, ${P_o}$ will cancel out from both sides. Thus, we get
$\Delta P{\text{ }} = {\text{ }} - {\text{ }}\rho gh{\text{ }} + {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Further, we get
$\Delta P{\text{ }} + {\text{ }}\rho gh{\text{ }} = {\text{ }}\dfrac{1}{2}{\text{ }}{\rho _a}{V^2}$
Then, we can write
$2{\text{ }}\left( {\Delta P{\text{ }} + {\text{ }}\rho gh} \right){\text{ }} = {\text{ }}{\rho _a}{V^2}$
Further, we get
$\dfrac{{2{\text{ }}\left( {\Delta P{\text{ }} + {\text{ }}\rho gh} \right)}}{{{\rho _a}}}{\text{ }} = {\text{ }}{V^2}$
Finally, we get
$\therefore V{\text{ }} = {\text{ }}\sqrt {\dfrac{{2{\text{ }}(\Delta P{\text{ }} + {\text{ }}\rho gh)}}{{{\rho _a}}}} $
Hence, the correct answer is B.
Additional information: Gauge pressure is the pressure which is excessive of the atmospheric pressure acting on a substance. Absolute pressure on a substance is simply the summation of the gauge pressure and the atmospheric pressure. This gauge pressure could be of any type. Let us say if an object is immersed in a liquid, then the gauge pressure here will be the pressure the liquid exerts on the walls of the object. Like this, the gauge pressure on an object will be dependent on the situation in which the object is kept in.
Note: Students should be very much cautious while substituting the value of gauge pressure as the gauge pressure is different in different cases. Students commit errors while manipulating the equations.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




