
The enolic form of acetone contains
(A) 9 sigma bonds, 1 pi bond, 2 lone pairs
(B) 8 sigma bonds, 2 pi bonds, 2 lone pairs
(C) 10 sigma bonds, 1 pi bond, 1 lone pair
(D) 9 sigma bonds, 2 pi bonds, 1 lone pair
Answer
589.2k+ views
Hint: We shall draw the structure of acetone and then draw its enol tautomer. Looking at the structure, we can determine the number of sigma bonds, pi bonds and lone pairs of electrons.
Complete step by step answer:
In organic reactions, due to the acidic nature of hydrogens present on the alpha carbon of ketones (that is hydrogen attached to the carbon atom next to the functional group – keep in mind that the carbon atom present adjacent to the functional group is known as alpha carbon atom), a reactive intermediate called enol is formed. As the name suggests, enol contains “ene”-double bond and “ol”-alcohol. There will be a chemical equilibrium established between the ketone form and its enol form. This is known as keto-enol tautomerism.
We know that the IUPAC name of acetone is propan-2-one and it has the structural formula
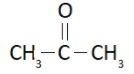
It forms an enol as shown below
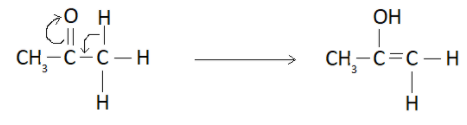
So the overall keto-enol tautomerism can be represented as
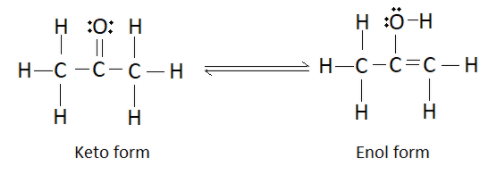
Now that we have the structure of the enol form, let us find the number of sigma and pi bonds and also the number of lone pairs of electrons. Just to recall, sigma bonds are the bond formed by axial overlap of atomic orbitals whereas pi bonds are formed by the lateral overlap. All the single bonds in the structure represent a sigma bond whereas the double bonds represent a pi bond.
Number of single bonds in the enol form of acetone is 9. Therefore there are 9 sigma bonds. Hence we can eliminate options (B) and (C). As you can observe in the structure, there is only one double bond which indicates the presence of one pi bond. So the correct answer is option (A). We can cross check the number of lone pairs of electrons as well. There are 2 lone pairs present on the oxygen atom.
Thus the correct answer is A).
Note: We know that a pi bond can be formed only after the formation of a sigma bond. So in a double bond, we should count one bond as sigma and the other as pi. Similarly, in a triple bond one bond will be sigma and the other two will be pi.
Complete step by step answer:
In organic reactions, due to the acidic nature of hydrogens present on the alpha carbon of ketones (that is hydrogen attached to the carbon atom next to the functional group – keep in mind that the carbon atom present adjacent to the functional group is known as alpha carbon atom), a reactive intermediate called enol is formed. As the name suggests, enol contains “ene”-double bond and “ol”-alcohol. There will be a chemical equilibrium established between the ketone form and its enol form. This is known as keto-enol tautomerism.
We know that the IUPAC name of acetone is propan-2-one and it has the structural formula
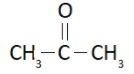
It forms an enol as shown below

So the overall keto-enol tautomerism can be represented as
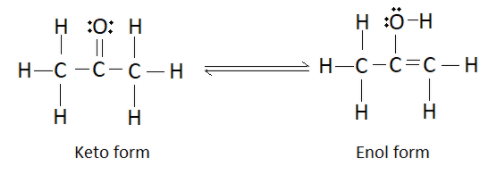
Now that we have the structure of the enol form, let us find the number of sigma and pi bonds and also the number of lone pairs of electrons. Just to recall, sigma bonds are the bond formed by axial overlap of atomic orbitals whereas pi bonds are formed by the lateral overlap. All the single bonds in the structure represent a sigma bond whereas the double bonds represent a pi bond.
Number of single bonds in the enol form of acetone is 9. Therefore there are 9 sigma bonds. Hence we can eliminate options (B) and (C). As you can observe in the structure, there is only one double bond which indicates the presence of one pi bond. So the correct answer is option (A). We can cross check the number of lone pairs of electrons as well. There are 2 lone pairs present on the oxygen atom.
Thus the correct answer is A).
Note: We know that a pi bond can be formed only after the formation of a sigma bond. So in a double bond, we should count one bond as sigma and the other as pi. Similarly, in a triple bond one bond will be sigma and the other two will be pi.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




