
What would be the electron dot structure of a molecule of a Sulphur which is made up of eight atoms of Sulphur?
Answer
614.7k+ views
Hint: Start the given question by drawing a structure in which the eight atoms of Sulphur are joined together in the form of a ring. Sulphur has six electrons in the valence shell. Draw the electrons and connect electrons of eight sulphur to form a compound.
Complete step by step answer:
Sulphur is a non-metallic element present in group 16 of the periodic table. It is a chalcogen.
The atomic number (Z) of Sulphur is sixteen. Its electronic configuration is 2, 8, 6. The spdf configuration is as follows –
\[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{4}}}\]
As we can see, Sulphur atom has six electrons in its valence shell. Therefore, it needs two more electrons to achieve a stable configuration, i.e. to complete its octet like its nearest noble gas – Argon.
According to the question, we need to find the electron dot structure of a molecule of Sulphur which is made up of eight atoms (of Sulphur).
Therefore, the chemical formula of such a compound will be \[{{\text{S}}_{\text{8}}}\]. In this, each sulphur atom is linked to other sulphur atoms on either side by single covalent bonds to complete its octet.
The electron dot structure can be represented in the following two ways –
The molecule is present in the form of a ring as given below –
(this is the top view)
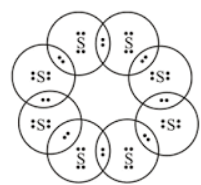
The molecule can also be represented by a crown shape, as given below –
(this is the side view)

Note: This compound - \[{{\text{S}}_{\text{8}}}\], is also known as Octasulfur. It occurs as a yellow solid that is odorless and tasteless. Octasulfur is the most common allotrope of sulfur. The molecular weight of this compound is 256.5 g/mol.
Complete step by step answer:
Sulphur is a non-metallic element present in group 16 of the periodic table. It is a chalcogen.
The atomic number (Z) of Sulphur is sixteen. Its electronic configuration is 2, 8, 6. The spdf configuration is as follows –
\[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{4}}}\]
As we can see, Sulphur atom has six electrons in its valence shell. Therefore, it needs two more electrons to achieve a stable configuration, i.e. to complete its octet like its nearest noble gas – Argon.
According to the question, we need to find the electron dot structure of a molecule of Sulphur which is made up of eight atoms (of Sulphur).
Therefore, the chemical formula of such a compound will be \[{{\text{S}}_{\text{8}}}\]. In this, each sulphur atom is linked to other sulphur atoms on either side by single covalent bonds to complete its octet.
The electron dot structure can be represented in the following two ways –
The molecule is present in the form of a ring as given below –
(this is the top view)

The molecule can also be represented by a crown shape, as given below –
(this is the side view)

Note: This compound - \[{{\text{S}}_{\text{8}}}\], is also known as Octasulfur. It occurs as a yellow solid that is odorless and tasteless. Octasulfur is the most common allotrope of sulfur. The molecular weight of this compound is 256.5 g/mol.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




