
The ${{d}_{{{x}^{2}}-{{y}^{2}}}}$ and ${{d}_{{{z}^{2}}}}$ orbitals are directed along with a set of mutually perpendicular x,y, and z-axis and are called ${{e}_{g}}$ orbitals. If true enter 1, or else enter 0.
Answer
596.1k+ views
Hint: Five degenerate d-orbitals of the central metal cation in the presence of four or six ligands in tetrahedral or octahedral complexes are assumed hypothetically that they undergo splitting into two degenerate orbitals, that is ${{t}_{2g}}$ and ${{e}_{g}}$ orbitals.
Complete step by step answer:
-The region in which the probability of finding an electron is the highest is known as an orbital. Smaller the size of the orbital means that there is a greater chance of getting an electron near the nucleus.
-There are four different types of orbitals: s-orbital; p-orbital; d-orbital and f-orbital.
-The magnetic quantum number values for d-orbitals are given as -2, -1, 0, 1, 2. Hence we can say that the d-orbital has five sub-orbitals.
-The five sub-orbitals of d-orbitals are designated as - ${{d}_{xy}},{{d}_{yz}},{{d}_{xz}},{{d}_{{{x}^{2}}-{{y}^{2}}}},\text{ and }{{d}_{{{z}^{2}}}}$ .
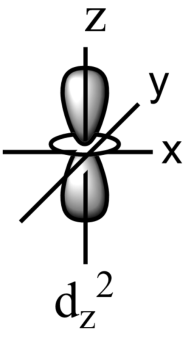
-Out of these five orbitals, the shapes \[{{d}_{xy}},{{d}_{yz}},{{d}_{xz}}\text{ and }{{\text{d}}_{{{x}^{2}}-{{y}^{2}}}}\]are similar to each other but are different from \[{{d}_{{{z}^{2}}}}\]sub-orbital, whereas the energy of all the fiver d-sub orbitals is the same.
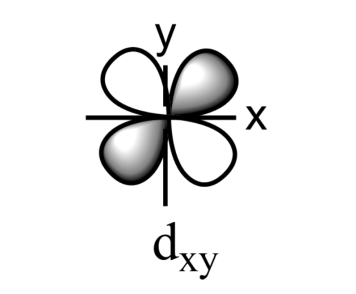
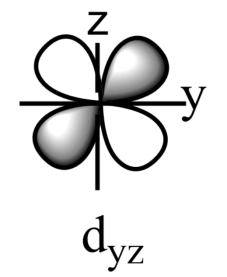
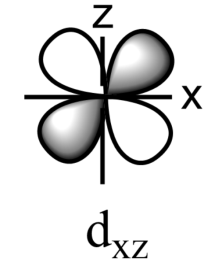
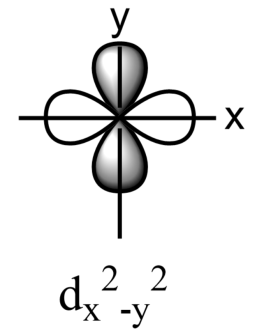
-The set of orbitals whose lobes lie in between the axis and are called non-axial orbitals. According to group theory, these non-axial orbitals are called ${{t}_{2g}}$ orbitals, where ‘t’ refers to the triply degenerate set.
-The set of orbitals whose lobes line along the axes are called axial orbitals. According to group theory, these axial orbitals are called ${{e}_{g}}$ orbitals where ‘e’ refers to the doubly degenerate set.
-${{d}_{xy}},{{d}_{yz,}}\text{ and }{{d}_{zx}}$ are called ${{t}_{2g}}$ set of orbitals, while ${{d}_{{{z}^{2}}}}\text{ and }{{d}_{{{x}^{2}}-{{y}^{2}}}}$ are ${{e}_{g}}$ set of orbitals.
-Hence, it is true that the ${{d}_{{{x}^{2}}-{{y}^{2}}}}$ and ${{d}_{{{z}^{2}}}}$ orbitals are directed along with a set of mutually perpendicular x,y, and z-axis.
So, the statement in the question is true.
Note: Crystal field theory is a theory that describes the breaking of degeneracies of electronic d-orbitals due to the static electric field produced by ligands. The electrons in the d-orbitals of the central metal atom in a coordination complex repel the electrons in the ligand due to the repulsion between the like charges. Thus the d-orbitals closer to the ligands attain higher energy while those away from the ligands attain the lower splitting energy.
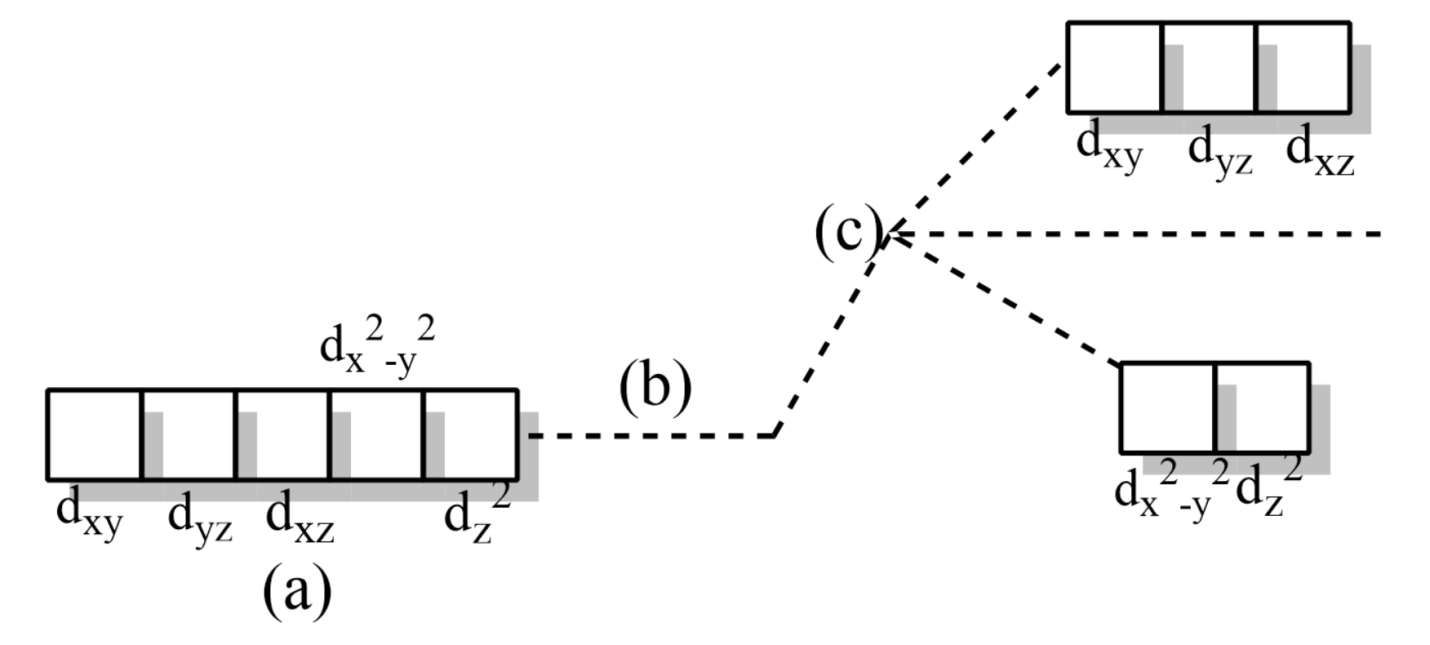
where,
(a) Five degenerate d-orbitals on the central metal cation before the approach of ligands
(b) Hypothetical degenerate d-orbitals at a higher energy level
(c) Splitting of degenerate d-orbitals into ${{t}_{2g}}$ and ${{e}_{g}}$ orbitals.
Complete step by step answer:
-The region in which the probability of finding an electron is the highest is known as an orbital. Smaller the size of the orbital means that there is a greater chance of getting an electron near the nucleus.
-There are four different types of orbitals: s-orbital; p-orbital; d-orbital and f-orbital.
-The magnetic quantum number values for d-orbitals are given as -2, -1, 0, 1, 2. Hence we can say that the d-orbital has five sub-orbitals.
-The five sub-orbitals of d-orbitals are designated as - ${{d}_{xy}},{{d}_{yz}},{{d}_{xz}},{{d}_{{{x}^{2}}-{{y}^{2}}}},\text{ and }{{d}_{{{z}^{2}}}}$ .
-Out of these five orbitals, the shapes \[{{d}_{xy}},{{d}_{yz}},{{d}_{xz}}\text{ and }{{\text{d}}_{{{x}^{2}}-{{y}^{2}}}}\]are similar to each other but are different from \[{{d}_{{{z}^{2}}}}\]sub-orbital, whereas the energy of all the fiver d-sub orbitals is the same.
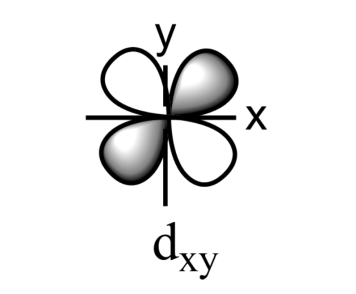
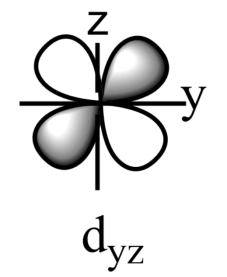
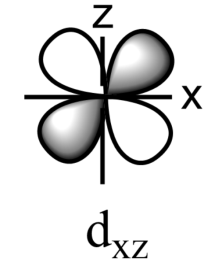
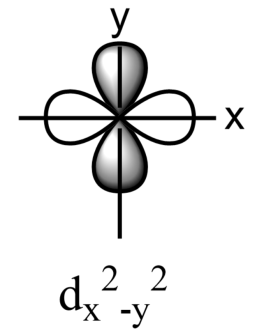

-The set of orbitals whose lobes lie in between the axis and are called non-axial orbitals. According to group theory, these non-axial orbitals are called ${{t}_{2g}}$ orbitals, where ‘t’ refers to the triply degenerate set.
-The set of orbitals whose lobes line along the axes are called axial orbitals. According to group theory, these axial orbitals are called ${{e}_{g}}$ orbitals where ‘e’ refers to the doubly degenerate set.
-${{d}_{xy}},{{d}_{yz,}}\text{ and }{{d}_{zx}}$ are called ${{t}_{2g}}$ set of orbitals, while ${{d}_{{{z}^{2}}}}\text{ and }{{d}_{{{x}^{2}}-{{y}^{2}}}}$ are ${{e}_{g}}$ set of orbitals.
-Hence, it is true that the ${{d}_{{{x}^{2}}-{{y}^{2}}}}$ and ${{d}_{{{z}^{2}}}}$ orbitals are directed along with a set of mutually perpendicular x,y, and z-axis.
So, the statement in the question is true.
Note: Crystal field theory is a theory that describes the breaking of degeneracies of electronic d-orbitals due to the static electric field produced by ligands. The electrons in the d-orbitals of the central metal atom in a coordination complex repel the electrons in the ligand due to the repulsion between the like charges. Thus the d-orbitals closer to the ligands attain higher energy while those away from the ligands attain the lower splitting energy.

where,
(a) Five degenerate d-orbitals on the central metal cation before the approach of ligands
(b) Hypothetical degenerate d-orbitals at a higher energy level
(c) Splitting of degenerate d-orbitals into ${{t}_{2g}}$ and ${{e}_{g}}$ orbitals.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




