
The dipole moments of $CC{l_4}$ , $CHC{l_3}$ and $C{H_4}$ are in the order :
A.$C{H_4} = CC{l_4} < CHC{l_3}$
B.$CC{l_4} < C{H_4} < CHC{l_3}$
C.$C{H_4} < CC{l_4} < CHC{l_3}$
D.$CHC{l_3} < C{H_4} = CC{l_4}$
Answer
590.1k+ views
Hint:The term dipole moment depends on various other concepts like polarity and pseudo charge. Apart from that the main factors affecting the dipole moment are difference in electrostatic charge and the structure of the given molecule. After making the structural diagrams for the given options we can observe that in among which of the diagrams there is least cancellation of the charges. And in among which we attain the most polarized ends. Then we can get the right answer.
Complete step by step answer:
Dipole moment is the phenomenon of the generation of the polarity in the structure of a specific structure of compound due to the generation of pseudo charge among the atoms of the molecule. This is mainly due to the difference in the electronegativity or electropositivity of various elements.
The generation of the dipole moment in a neutral environment is mainly due to $2$ reasons:
i.The first one is the difference in the electronegativity or electropositivity
ii.And the second one is the structure of the molecule.
For. Eg: the hydrogen would have no dipole moment due to the linear structure and no difference in electronegativity .
Now regarding the options :
Option 1: $CC{l_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
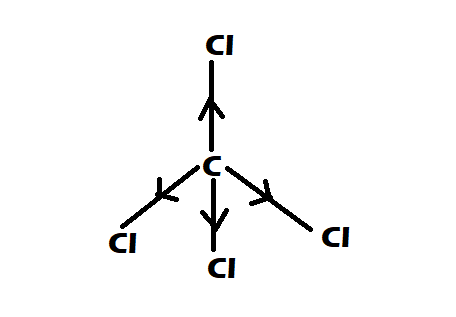
Option 2: $CHC{l_3}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
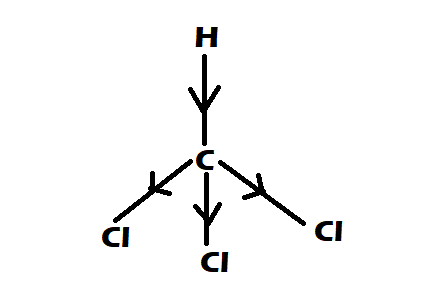
Option 3: $C{H_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
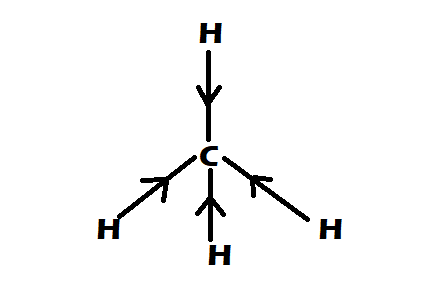
Therefore the correct option would be option A, $C{H_4} = CC{l_4} < CHC{l_3}$ .
Note:Despite having the same atoms connected to the element many times the dipole moment don’t be null. This happens due to the change in the structure in which the charges don't cancel each other and this happens due to the presence of the lone pair.
Complete step by step answer:
Dipole moment is the phenomenon of the generation of the polarity in the structure of a specific structure of compound due to the generation of pseudo charge among the atoms of the molecule. This is mainly due to the difference in the electronegativity or electropositivity of various elements.
The generation of the dipole moment in a neutral environment is mainly due to $2$ reasons:
i.The first one is the difference in the electronegativity or electropositivity
ii.And the second one is the structure of the molecule.
For. Eg: the hydrogen would have no dipole moment due to the linear structure and no difference in electronegativity .
Now regarding the options :
Option 1: $CC{l_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Option 2: $CHC{l_3}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Option 3: $C{H_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Therefore the correct option would be option A, $C{H_4} = CC{l_4} < CHC{l_3}$ .
Note:Despite having the same atoms connected to the element many times the dipole moment don’t be null. This happens due to the change in the structure in which the charges don't cancel each other and this happens due to the presence of the lone pair.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




