
The diagram shows a U-tube manometer containing three liquids: mercury, liquid X and liquid Y. Neither liquid X nor liquid Y mixes with mercury. Which row compared the pressure exerted by liquid X and by liquid Y on the mercury, and the density of liquid X and the density of liquid Y?
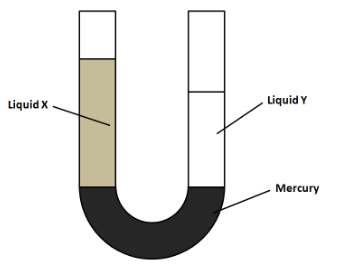
(A) Pressure exerted by X and by Y on the mercury – pressure of X is greater than Y. Densities of X and Y – density of X is greater than Y.
(B) Pressure exerted by X and by Y on the mercury – pressure of X is greater than Y. Densities of X and Y – density of Y is greater than X.
(C) Pressure exerted by X and by Y on the mercury – pressure of X and Y is the same. Densities of X and Y – density of X is greater than Y.
(D) Pressure exerted by X and by Y on the mercury – pressure of X and Y is the same. Densities of X and Y – density of Y is greater than X.

Answer
570.9k+ views
Hint : To solve this question, we have to use Pascal's law for the pressure inside a fluid contained in a vessel. Using this we can find out the required relations between the pressures exerted on the mercury and the densities of the two liquids.
Formula Used: The formula which is used in solving this question is given by
$\Rightarrow P = {P_o} + \rho gh $ , here $ P $ is the pressure of a fluid column of density $ \rho $ at a point which is situated at a depth of $ h $ , and $ {P_0} $ is the atmospheric pressure.
Complete step by step answer
Let $ {h_X} $ and $ {h_Y} $ be the heights of the liquids columns X and Y respectively. Also, let $ {\rho _X} $ and $ {\rho _Y} $ be the respective densities. For the pressure exerted on the mercury, we consider the two points A and B as shown in the figure below.
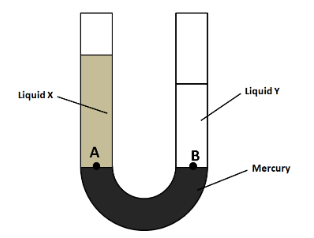
We know from Pascal's law that the pressures of a fluid at the points which are located at the same level are equal. So we have
$\Rightarrow {P_A} = {P_B} $
Therefore, the pressure exerted by the liquids X and Y on the mercury is the same. Now, we know that the pressure of a fluid column can be written as
$\Rightarrow P = {P_o} + \rho gh $
So from
$ \Rightarrow {P_o} + {\rho _Y}g{h_Y} = {P_o} + {\rho _X}g{h_X} $
Subtracting $ {P_0} $ from both the sides, we get
$\Rightarrow {\rho _Y}g{h_Y} = {\rho _X}g{h_X} $
Dividing by $ g $ both sides
$\Rightarrow {\rho _Y}{h_Y} = {\rho _X}{h_X} $
Dividing by $ {\rho _X}{h_Y} $ both sides, we have
$\Rightarrow \dfrac{{{\rho _Y}}}{{{\rho _X}}} = \dfrac{{{h_X}}}{{{h_Y}}} $ ……………………….(i)
From the figure in the above figure, we can clearly see that $ {h_X} > {h_Y} $ . So we have
$\Rightarrow \dfrac{{{h_X}}}{{{h_Y}}} > 1 $
From (i) we get
$\Rightarrow \dfrac{{{\rho _Y}}}{{{\rho _X}}} > 1 $
Or
$\Rightarrow {\rho _Y} > {\rho _X} $
So the density of the liquid Y is greater than that of the liquid X.
Hence, the correct answer is option D.
Note
We should not try to compare the densities using the contrast of the colors in the diagram given in the question. The diagram may be represented anyway, but the final comparison will only be based on the analytical calculations only.
Formula Used: The formula which is used in solving this question is given by
$\Rightarrow P = {P_o} + \rho gh $ , here $ P $ is the pressure of a fluid column of density $ \rho $ at a point which is situated at a depth of $ h $ , and $ {P_0} $ is the atmospheric pressure.
Complete step by step answer
Let $ {h_X} $ and $ {h_Y} $ be the heights of the liquids columns X and Y respectively. Also, let $ {\rho _X} $ and $ {\rho _Y} $ be the respective densities. For the pressure exerted on the mercury, we consider the two points A and B as shown in the figure below.

We know from Pascal's law that the pressures of a fluid at the points which are located at the same level are equal. So we have
$\Rightarrow {P_A} = {P_B} $
Therefore, the pressure exerted by the liquids X and Y on the mercury is the same. Now, we know that the pressure of a fluid column can be written as
$\Rightarrow P = {P_o} + \rho gh $
So from
$ \Rightarrow {P_o} + {\rho _Y}g{h_Y} = {P_o} + {\rho _X}g{h_X} $
Subtracting $ {P_0} $ from both the sides, we get
$\Rightarrow {\rho _Y}g{h_Y} = {\rho _X}g{h_X} $
Dividing by $ g $ both sides
$\Rightarrow {\rho _Y}{h_Y} = {\rho _X}{h_X} $
Dividing by $ {\rho _X}{h_Y} $ both sides, we have
$\Rightarrow \dfrac{{{\rho _Y}}}{{{\rho _X}}} = \dfrac{{{h_X}}}{{{h_Y}}} $ ……………………….(i)
From the figure in the above figure, we can clearly see that $ {h_X} > {h_Y} $ . So we have
$\Rightarrow \dfrac{{{h_X}}}{{{h_Y}}} > 1 $
From (i) we get
$\Rightarrow \dfrac{{{\rho _Y}}}{{{\rho _X}}} > 1 $
Or
$\Rightarrow {\rho _Y} > {\rho _X} $
So the density of the liquid Y is greater than that of the liquid X.
Hence, the correct answer is option D.
Note
We should not try to compare the densities using the contrast of the colors in the diagram given in the question. The diagram may be represented anyway, but the final comparison will only be based on the analytical calculations only.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




