
The diagram shows a molecule hydrogen fluoride,
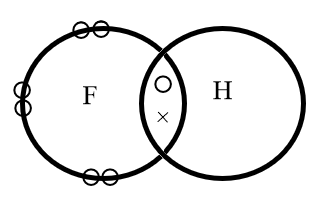
In the molecule,
A.The hydrogen and fluorine share a pair of electrons
B.The hydrogen and fluorine share a pair of protons
C.The hydrogen gives the fluorine an electron
D.The hydrogen gives fluorine a proton

Answer
516.9k+ views
Hint: The electron dot diagram or Lewis dot diagram is a structural representation of a molecule, in which the dots outside the common part represent a lone pair of electrons whereas dots inside the common part of the structure represent the number of electrons participating in the bond formation.
Complete answer:
The $HF$ molecule consist of a hydrogen and a fluorine atom whose electron configurations are given as follows:
Atomic number of Hydrogen $ = 2$
Electronic configuration $ = 1{s^2}$
Atomic number of fluorine $ = 9$
Electronic configuration $ = 1{s^2}2{s^2}2{p^5}$
As there is only one electron in the valence shell of hydrogen atom and there are seven electrons present in the valence shell of fluorine atom. So, in order to complete the valency or octet of each atom, both the atoms perform mutual sharing of their electrons and hence formation of a covalent bond takes place between the atoms.
The electron dot structure of hydrogen fluoride is represented as follows:
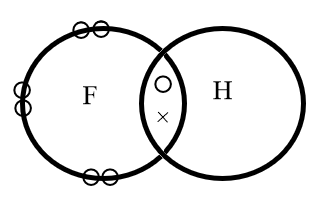
Where, circular electrons on the edge of a sphere represents the lone pair of electrons whereas the electrons represented with a circle and a cross represents the bond formed by mutual sharing of electrons.
Hence, in the molecule the hydrogen and fluorine share a pair of electrons.
Thus, option (A) is the correct answer.
Note:
According to octet rule, the main group elements tend to form a chemical bond in such a manner that the octet of the atom is completed i.e., each atom must have eight electrons in its valence shell after the formation of the bond.
Complete answer:
The $HF$ molecule consist of a hydrogen and a fluorine atom whose electron configurations are given as follows:
Atomic number of Hydrogen $ = 2$
Electronic configuration $ = 1{s^2}$
Atomic number of fluorine $ = 9$
Electronic configuration $ = 1{s^2}2{s^2}2{p^5}$
As there is only one electron in the valence shell of hydrogen atom and there are seven electrons present in the valence shell of fluorine atom. So, in order to complete the valency or octet of each atom, both the atoms perform mutual sharing of their electrons and hence formation of a covalent bond takes place between the atoms.
The electron dot structure of hydrogen fluoride is represented as follows:

Where, circular electrons on the edge of a sphere represents the lone pair of electrons whereas the electrons represented with a circle and a cross represents the bond formed by mutual sharing of electrons.
Hence, in the molecule the hydrogen and fluorine share a pair of electrons.
Thus, option (A) is the correct answer.
Note:
According to octet rule, the main group elements tend to form a chemical bond in such a manner that the octet of the atom is completed i.e., each atom must have eight electrons in its valence shell after the formation of the bond.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




