
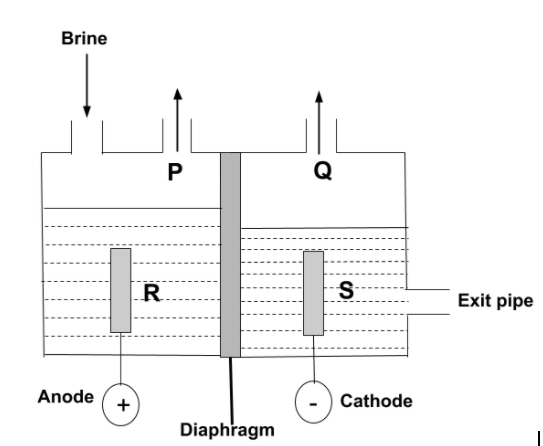
The diagram shows a diaphragm cell used for the electrolysis of brine. Brine is concentrated aqueous sodium chloride. A solution of sodium chlorate (I) which is commonly used as bleach, can be made by mixing which of the two substances?
A.P and R
B.P and S
C.Q and R
D.Q and S

Answer
575.4k+ views
Hint:The chemical formula for sodium chlorate is $NaCl{O_3}$ which can be formed by the reaction between chloride and sodium hydroxide. Brine is a high concentration solution of sodium chloride salt in the water.
Complete step by step answer:
-The high concentration solution of salt sodium chloride $NaCl$ in water, ${H_2}O$ is called Brine. Lower levels of concentration can be called by other names: saline water, freshwater and brackish water.
-Sodium chlorate is the white crystalline powder which is readily soluble in water and is the inorganic compound with the molecular formula, $NaCl{O_3}$ .
-Now, according to the question, the electrolysis of brine is carried out. So, we have to find which substances are used for the formation of sodium chlorate, $NaCl{O_3}$. In this electrolysis, the diaphragm cell process is used in which there are two compartments separated by permeable diaphragm which is generally made of asbestos fibers. Brine is introduced into the anode and flows into the cathode compartment.
-The chloride ion is negatively charged, so it can get attracted towards positively charged anode. So, P can be $C{l_2}$ , whereas sodium ions and hydrogen ions from the water are positively charged so, they get attracted towards negative cathode, then, the dilute solution of sodium hydroxide is produced at cathode, so, S can be $NaOH$. Hence, the reaction is –
$6NaOH + 3C{l_2}\xrightarrow[{}]{}NaC{l_3} + 5NaCl + 3{H_2}O$
-Therefore, the sodium chlorate solution can be made by mixing P and S.
Hence, the correct option is (B).
Note:
When the electrolysis of aqueous $NaCl$ is carried out we have to take water into equation. Water can be oxidized and reduced both and it competes with the dissolved $N{a^ + }$ and $C{l^ - }$ ions. This produces hydrogen instead of producing sodium.
Complete step by step answer:
-The high concentration solution of salt sodium chloride $NaCl$ in water, ${H_2}O$ is called Brine. Lower levels of concentration can be called by other names: saline water, freshwater and brackish water.
-Sodium chlorate is the white crystalline powder which is readily soluble in water and is the inorganic compound with the molecular formula, $NaCl{O_3}$ .
-Now, according to the question, the electrolysis of brine is carried out. So, we have to find which substances are used for the formation of sodium chlorate, $NaCl{O_3}$. In this electrolysis, the diaphragm cell process is used in which there are two compartments separated by permeable diaphragm which is generally made of asbestos fibers. Brine is introduced into the anode and flows into the cathode compartment.
-The chloride ion is negatively charged, so it can get attracted towards positively charged anode. So, P can be $C{l_2}$ , whereas sodium ions and hydrogen ions from the water are positively charged so, they get attracted towards negative cathode, then, the dilute solution of sodium hydroxide is produced at cathode, so, S can be $NaOH$. Hence, the reaction is –
$6NaOH + 3C{l_2}\xrightarrow[{}]{}NaC{l_3} + 5NaCl + 3{H_2}O$
-Therefore, the sodium chlorate solution can be made by mixing P and S.
Hence, the correct option is (B).
Note:
When the electrolysis of aqueous $NaCl$ is carried out we have to take water into equation. Water can be oxidized and reduced both and it competes with the dissolved $N{a^ + }$ and $C{l^ - }$ ions. This produces hydrogen instead of producing sodium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




