
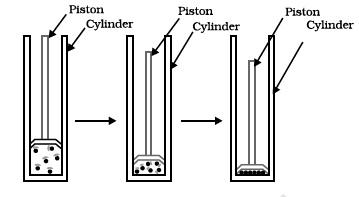
The diagram given shows a piston fitted in a cylinder. The most suitable inference that can be drawn from it is that _____________.
A.the piston is smooth
B.the particles can be compressed or brought closer by applying pressure
C.the handle of piston is long
D.None of the above

Answer
561.6k+ views
Hint:We know that by increasing the pressure of particles in a closed space can be brought about by reducing volume and hence they would get close together. In cases of flow, it would cause a decrease in velocity. So, when the pressure increases, the mean free path of particles is reduced and hence they would get closer together.
Complete answer:
Compressibility is the measure of how much a given volume of matter decreases when placed under pressure. If we put pressure on a solid or a liquid, there is essentially no change in volume as the atoms, ions, or the molecules from which the solid or liquid are made up are very close together and there is no space between the individual particles, so they cannot pack together.
Gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. At room temperature and standard pressure, the average distance between gas molecules is about ten times the diameter of the molecules themselves. When a gas is compressed, as when the scuba tank is being filled, the gas particles are forced closer together.
In the given picture, we can see the piston is pressured further in the cylinder. So, it means that the particles come closer as we push the piston down i.e. by applying pressure on the particles.
Therefore, the correct answer is option (B).
Note:
It should be noted that gases will compress more easily than solids or liquids because here is so much space between the gas molecules.The kinetic-molecular theory explains why gases are more compressible than either liquids or solids.
Complete answer:
Compressibility is the measure of how much a given volume of matter decreases when placed under pressure. If we put pressure on a solid or a liquid, there is essentially no change in volume as the atoms, ions, or the molecules from which the solid or liquid are made up are very close together and there is no space between the individual particles, so they cannot pack together.
Gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. At room temperature and standard pressure, the average distance between gas molecules is about ten times the diameter of the molecules themselves. When a gas is compressed, as when the scuba tank is being filled, the gas particles are forced closer together.
In the given picture, we can see the piston is pressured further in the cylinder. So, it means that the particles come closer as we push the piston down i.e. by applying pressure on the particles.
Therefore, the correct answer is option (B).
Note:
It should be noted that gases will compress more easily than solids or liquids because here is so much space between the gas molecules.The kinetic-molecular theory explains why gases are more compressible than either liquids or solids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




