
The correct structural formula of propanoic acid is:
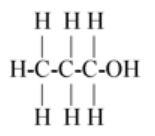
$\left( A \right)C{H_3}C{H_2}C{H_2}OH$
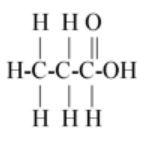
$\left( B \right)C{H_3}C{H_2}COOH$
$\left( C \right)C{H_2}CHCOOH$
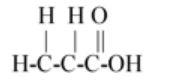
$\left( D \right)C{H_3}C{H_2}C{H_2}COOH$
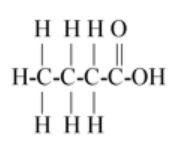


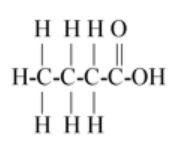
Answer
599.1k+ views
Hint – In this question use the molecular formula of propanoic acid which is ${C_3}{H_6}{O_2}$ or $C{H_3}C{H_2}COOH$. This concept along with the basics of carbon that only 4 outermost valence electrons are present in carbon and this needs to be satisfied for every carbon atom will help in getting the right structural formula.
Complete step by step answer:
As we know that the molecular formula or chemical formula of propanoic acid is
${C_3}{H_6}{O_2}$ or $C{H_3}C{H_2}COOH$.
So in propanoic acid there are 3 atoms of carbon 6 atoms of hydrogen and 2 atoms of oxygen.
As we know that the valence electrons in carbon is 4, in hydrogen is 1 and in oxygen is 2.
So first carbon bond with 3 hydrogen and 1 carbon so first carbon valence electrons are filled and second carbon remains 3 valence electrons (as 1 electron is paired with first carbon) so the next carbon has 3 valence electrons so it bonds with next two hydrogen and one with third carbon so the third carbon has 3 valence electrons so it paired its two electrons with one oxygen and one electron with another oxygen so oxygen remains one valence electron so it bond with the last hydrogen.
The above description is shown in option (B).
Hence option (B) is the correct answer.
Note – Structural formula is a chemical formula showing the composition and the structure of a molecule. The atoms are represented by symbols and the structure is indicated by showing the relative positions of the atoms in space and the bonds between them. It is different from empirical formula as empirical formula shows the simplest whole number ratio of atoms in a compound.
Complete step by step answer:
As we know that the molecular formula or chemical formula of propanoic acid is
${C_3}{H_6}{O_2}$ or $C{H_3}C{H_2}COOH$.
So in propanoic acid there are 3 atoms of carbon 6 atoms of hydrogen and 2 atoms of oxygen.
As we know that the valence electrons in carbon is 4, in hydrogen is 1 and in oxygen is 2.
So first carbon bond with 3 hydrogen and 1 carbon so first carbon valence electrons are filled and second carbon remains 3 valence electrons (as 1 electron is paired with first carbon) so the next carbon has 3 valence electrons so it bonds with next two hydrogen and one with third carbon so the third carbon has 3 valence electrons so it paired its two electrons with one oxygen and one electron with another oxygen so oxygen remains one valence electron so it bond with the last hydrogen.
The above description is shown in option (B).
Hence option (B) is the correct answer.
Note – Structural formula is a chemical formula showing the composition and the structure of a molecule. The atoms are represented by symbols and the structure is indicated by showing the relative positions of the atoms in space and the bonds between them. It is different from empirical formula as empirical formula shows the simplest whole number ratio of atoms in a compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




