
The correct order of stability of following carbocation is:
(I)
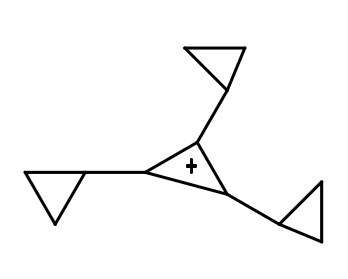
(II)
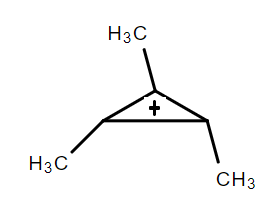
(III)
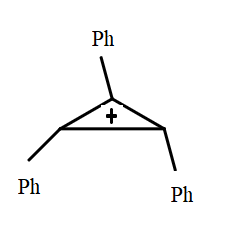
(IV)
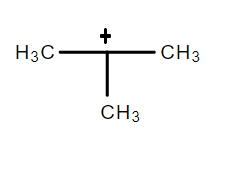
(A) $ {\text{(III) > (II) > (I) > (IV)}} $
(B) $ {\text{(II) > (III) > (I) > (IV)}} $
(C) $ {\text{(I) > (III) > (IV) > (II)}} $
(D) $ {\text{(I) > (III) > (II) > (IV)}} $




Answer
548.4k+ views
Hint: To answer this question, you should recall the concept of resonance and inductive effect and how they affect the stability of carbocation. The more positive charge is neutralized, more will be the stability.
Complete step by step solution:
The resonance effect can be defined as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect. An electron withdrawing group stabilizes a negative charge and an electron donating group stabilizes a positive charge. Dancing resonance is a special stability mechanism which increases stability of carbocations attached directly to the three membered rings for example, cyclopropyl carbocation. In cyclopropane all the carbon is $ s{p^3} $ hybridized and the bond angle for the same should be $ {109^o}28\prime \; $ but the actual bond angle is $ {60^o} $ due to which angle strain develops. So in order to minimize the strain p orbital bents due to which it acquires partial sigma and partial pi bond character which behave like pi bonds. And resonance takes place between sigma and vacant p-orbitals hence called P-orbitals overlapping or sigma resonance. The exceptional stability of cyclopropane methyl cation can be explained by the concept of dancing resonance concept. The stability of additional cyclopropyl groups is the result of more conjugation between the bent orbital of the cyclopropyl ring and cationic carbon. Compared to cyclopropyl groups, phenyl groups give less stability.
Hence, the correct option is option D.
Note:
You should know about the concept of stainless rings. The idea was given by the chemist Adolf von Baeyer that the stability of carbocyclic compounds. Carbocyclic compounds are defined as the compounds those of which the molecular structure includes one or more rings of carbon atoms) depends on the amount by which the angles between the chemical bonds deviate from the value $ \left( {109^\circ 28\prime } \right) $ observed in compounds not containing such rings. The amount of deviation is calculated from the strain to assess the stability of the ring: the greater the strain, the less stable is the ring. He gave the idea that these carbocyclic rings are planar and concluded that strain exists in three- and four-membered rings and in rings of six or more atoms, the strain increasing with the size of the ring. The least strained will be observed in the ring containing five carbon atoms which is cyclopentane, and the bond angles are $ 108^\circ . $
Complete step by step solution:
The resonance effect can be defined as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect. An electron withdrawing group stabilizes a negative charge and an electron donating group stabilizes a positive charge. Dancing resonance is a special stability mechanism which increases stability of carbocations attached directly to the three membered rings for example, cyclopropyl carbocation. In cyclopropane all the carbon is $ s{p^3} $ hybridized and the bond angle for the same should be $ {109^o}28\prime \; $ but the actual bond angle is $ {60^o} $ due to which angle strain develops. So in order to minimize the strain p orbital bents due to which it acquires partial sigma and partial pi bond character which behave like pi bonds. And resonance takes place between sigma and vacant p-orbitals hence called P-orbitals overlapping or sigma resonance. The exceptional stability of cyclopropane methyl cation can be explained by the concept of dancing resonance concept. The stability of additional cyclopropyl groups is the result of more conjugation between the bent orbital of the cyclopropyl ring and cationic carbon. Compared to cyclopropyl groups, phenyl groups give less stability.
Hence, the correct option is option D.
Note:
You should know about the concept of stainless rings. The idea was given by the chemist Adolf von Baeyer that the stability of carbocyclic compounds. Carbocyclic compounds are defined as the compounds those of which the molecular structure includes one or more rings of carbon atoms) depends on the amount by which the angles between the chemical bonds deviate from the value $ \left( {109^\circ 28\prime } \right) $ observed in compounds not containing such rings. The amount of deviation is calculated from the strain to assess the stability of the ring: the greater the strain, the less stable is the ring. He gave the idea that these carbocyclic rings are planar and concluded that strain exists in three- and four-membered rings and in rings of six or more atoms, the strain increasing with the size of the ring. The least strained will be observed in the ring containing five carbon atoms which is cyclopentane, and the bond angles are $ 108^\circ . $
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




