
The correct order of reactivity of the following compounds towards Grignard reagent
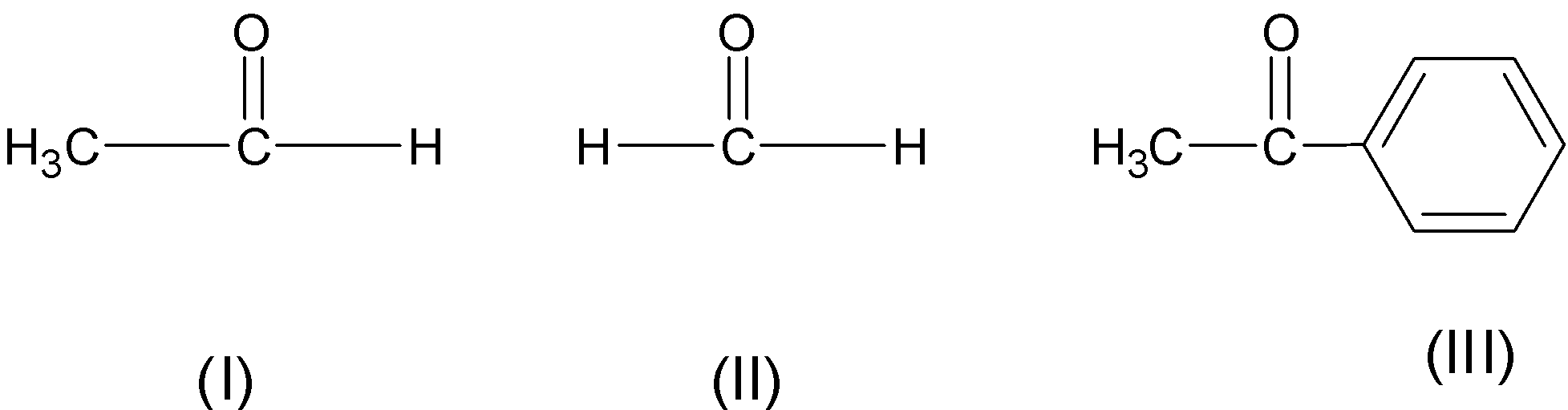
A) \[\text{I }\rangle \text{ II }\rangle \text{ III}\]
B) \[\text{II }\rangle \text{ I }\rangle \text{ III}\]
C) \[\text{II }\rangle \text{ III }\rangle \text{ I}\]
D) \[\text{I }\rangle \text{ III }\rangle \text{ II}\]

Answer
589.5k+ views
Hint: The order of reactivity of nucleophile towards the nucleophilic substitution reaction depends on the steric hindrance of the carbonyl compound. The higher the number of bulky groups at the carbonyl group less positive charge acquired by the carbon of carbonyl group and less is the reactivity of the compound towards the nucleophile i.e Grignard reagent.
Complete step by step answer:
Grignard reagents have the general formula $\text{ RMgX}$ where X is a halogen and R is the alkyl group. It is an organometallic compound. An alkyl halide with the electrophilic group is converted into its nucleophilic analog. Grignard reagents are nucleophile thus they undergo the nucleophilic substitution reaction with the organic compounds.
In the nucleophilic reaction of the Grignard reaction, the magnesium atom is transferred to the more electronegative atom. Like this the Grignard reagent acts on the carbonyl group of the aldehyde and ketone, where the negative group of the Grignard reagent attaches to the positive charge carbon atom.
The general reaction of the Grignard reagent with the carbonyl compound is as shown below:
$\text{R-CHO + R }\!\!'\!\!\text{ MgBr }\to \text{ R-C(OH)R }\!\!'\!\!\text{ + MgOBr}$
The reactivity of Grignard reagent changes with the type of aldehyde.
Aldehydes with the less bulky group at the R group have a high rate of reaction towards the Grignard reagent. Thus the aldehyde with the hydrogen as the group is less bulky than the aldehyde having methyl group as a substituent. Thus reactivity of the Grignard reagent is more reactive towards the formaldehyde than ethanol.
$\text{ HCHO }\rangle \text{ C}{{\text{H}}_{\text{3}}}\text{CHO}$
Let's compare the reactivity of Grignard reagent toward aldehyde and ketone. Aldehydes are more reactive towards the Grignard reagent or the nucleophilic substitution reaction than the ketone. The reason is as follows:
1) In aldehyde$\text{(R-CHO)}$, the carbonyl group $\text{(C=O)}$ is attached to a relatively small number of hydrogen at the one side of the carbonyl group. The larger R group is on the other side of the carbonyl group. However, in ketone R groups are attached to either side of the carbonyl group. Therefore, the steric hindrance in ketone is greater than aldehyde. Thus reactivity of nucleophiles is less towards the ketone than aldehyde.
2) The aldehyde has only a single R group which supplies the electrons to the partially positively charged carbon atom. However, in ketone, there are two electron-donating groups on either side of the carbonyl group. The greater the number of electrons to the carbonyl group less is the positive charge and the weaker the nucleus. Thus aldehyde has a more positive charge and thus more rate of reaction.
$\text{ Aldehyde }\rangle \text{ Ketone}$
Therefore, the acetophenone is less reactive than ethanal which is less reactive than formaldehyde.
$\text{ HCHO }\rangle \text{ C}{{\text{H}}_{\text{3}}}\text{CHO }\rangle \text{ Ph-CO-C}{{\text{H}}_{\text{3}}}$
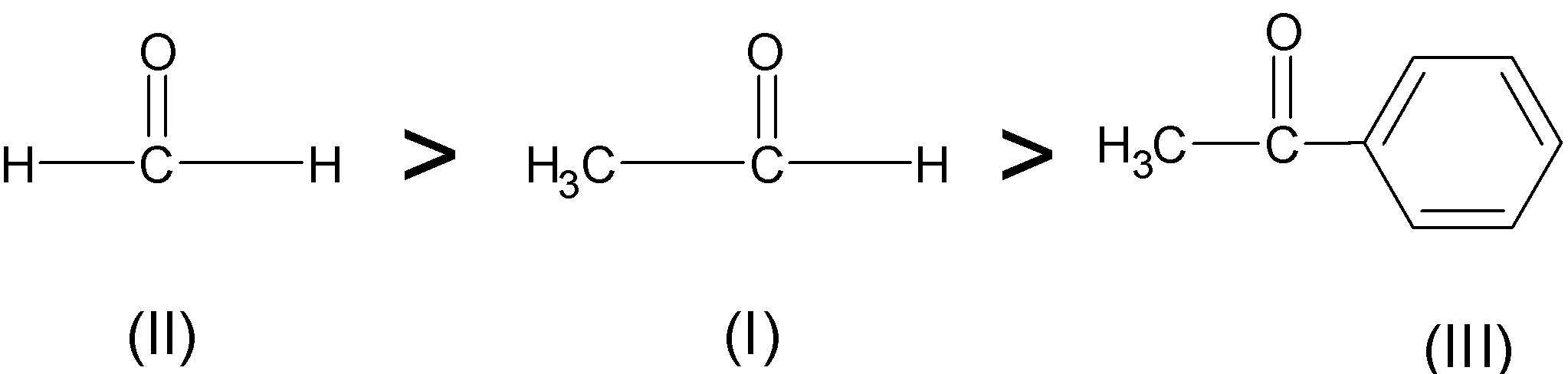
Therefore, the correct order of reactivity is: \[\text{II }\rangle \text{ I }\rangle \text{ III}\]
So, the correct answer is “Option B”.
Note: We may think that ester is more reactive toward Grignard reagent. But that’s not true: the carbonyl group of the ester is less electrophilic since the oxygen donates the electron pair to the carbon make is less reactive toward nucleophilic substitution reaction.
Complete step by step answer:
Grignard reagents have the general formula $\text{ RMgX}$ where X is a halogen and R is the alkyl group. It is an organometallic compound. An alkyl halide with the electrophilic group is converted into its nucleophilic analog. Grignard reagents are nucleophile thus they undergo the nucleophilic substitution reaction with the organic compounds.
In the nucleophilic reaction of the Grignard reaction, the magnesium atom is transferred to the more electronegative atom. Like this the Grignard reagent acts on the carbonyl group of the aldehyde and ketone, where the negative group of the Grignard reagent attaches to the positive charge carbon atom.
The general reaction of the Grignard reagent with the carbonyl compound is as shown below:
$\text{R-CHO + R }\!\!'\!\!\text{ MgBr }\to \text{ R-C(OH)R }\!\!'\!\!\text{ + MgOBr}$
The reactivity of Grignard reagent changes with the type of aldehyde.
Aldehydes with the less bulky group at the R group have a high rate of reaction towards the Grignard reagent. Thus the aldehyde with the hydrogen as the group is less bulky than the aldehyde having methyl group as a substituent. Thus reactivity of the Grignard reagent is more reactive towards the formaldehyde than ethanol.
$\text{ HCHO }\rangle \text{ C}{{\text{H}}_{\text{3}}}\text{CHO}$
Let's compare the reactivity of Grignard reagent toward aldehyde and ketone. Aldehydes are more reactive towards the Grignard reagent or the nucleophilic substitution reaction than the ketone. The reason is as follows:
1) In aldehyde$\text{(R-CHO)}$, the carbonyl group $\text{(C=O)}$ is attached to a relatively small number of hydrogen at the one side of the carbonyl group. The larger R group is on the other side of the carbonyl group. However, in ketone R groups are attached to either side of the carbonyl group. Therefore, the steric hindrance in ketone is greater than aldehyde. Thus reactivity of nucleophiles is less towards the ketone than aldehyde.
2) The aldehyde has only a single R group which supplies the electrons to the partially positively charged carbon atom. However, in ketone, there are two electron-donating groups on either side of the carbonyl group. The greater the number of electrons to the carbonyl group less is the positive charge and the weaker the nucleus. Thus aldehyde has a more positive charge and thus more rate of reaction.
$\text{ Aldehyde }\rangle \text{ Ketone}$
Therefore, the acetophenone is less reactive than ethanal which is less reactive than formaldehyde.
$\text{ HCHO }\rangle \text{ C}{{\text{H}}_{\text{3}}}\text{CHO }\rangle \text{ Ph-CO-C}{{\text{H}}_{\text{3}}}$

Therefore, the correct order of reactivity is: \[\text{II }\rangle \text{ I }\rangle \text{ III}\]
So, the correct answer is “Option B”.
Note: We may think that ester is more reactive toward Grignard reagent. But that’s not true: the carbonyl group of the ester is less electrophilic since the oxygen donates the electron pair to the carbon make is less reactive toward nucleophilic substitution reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




