
The correct order of decreasing acid strength of trichloroacetic acid (A), trifluoroacetic acid (B), acetic acid (C) and formic acid (D) is:
A) \[A > B > C > D\]
B) \[A > C > B > D\]
C) \[B > A > D > C\]
D) \[B > D > C > A\]
Answer
535.8k+ views
Hint: An acid can be defined as a chemical which releases hydrogen ions in water thereby forming salts by integrating with specific metals. \[pH\] scale can be used to measure acidity. On the pH scale, value of 7 is considered to be neutral, whereas, a value from 7 to 0 indicates increasing acidity.
Complete step by step answer:
In general, the acidic strength in an organic compound is directly related to the stability of the acid's conjugate base. In other words, an acid having a more stable conjugate base will be more acidic in comparison to an acid possessing a less stable conjugate base. In the given question, all four given acids are carboxylic acid ($ - COOH$) whose conjugate base ($ - CO{O^ - }$) includes a negative charge. This conjugate base can be stabilized if its carbon gets connected to an electron withdrawing group. Now let us look at the four given acids one by one:
(A) Trichloroacetic acid: The structure is given below:
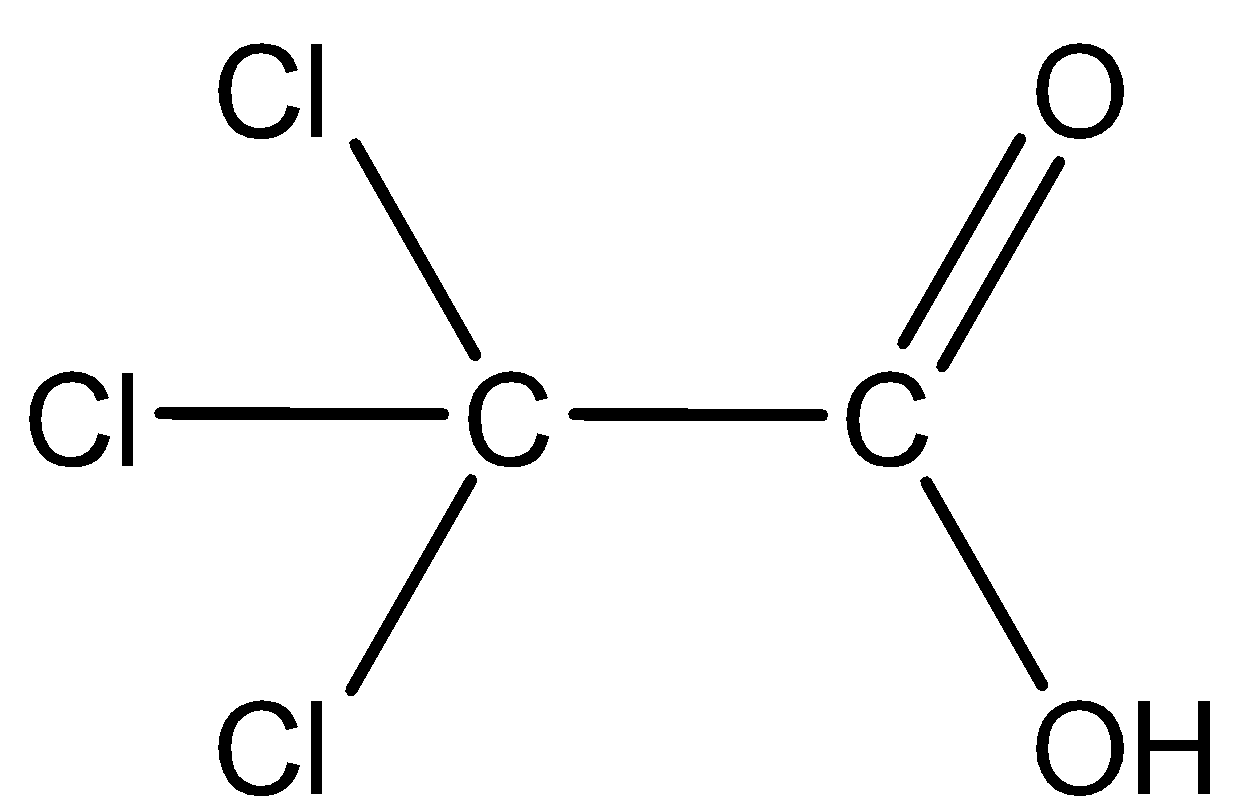
Here three chloride groups attached are the electron withdrawing groups which impose the\[-I\] effect thereby increasing the acidic strength. Thus, trichloroacetic acid is highly acidic.
(B) Trifluoroacetic acid: The structure is given below:
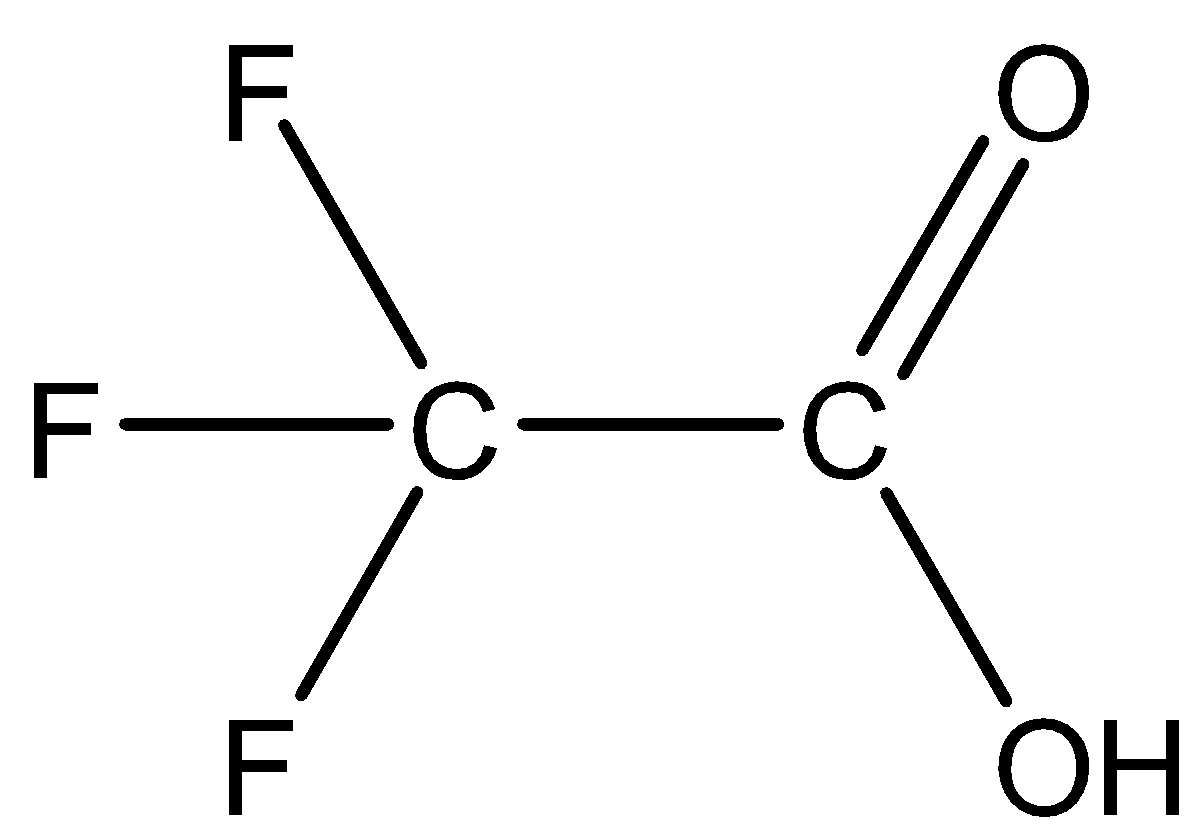
Here three fluoride groups attached are the electron withdrawing groups which impose the\[-I\] effect thereby increasing the acidic strength. Thus, trifluoroacetic acid is highly acidic. Since\[-I\] effect of fluorine is more than that of chlorine, trifluoroacetic acid is more acidic in comparison to trichloroacetic acid.
(C) Acetic acid: The structure is given below:
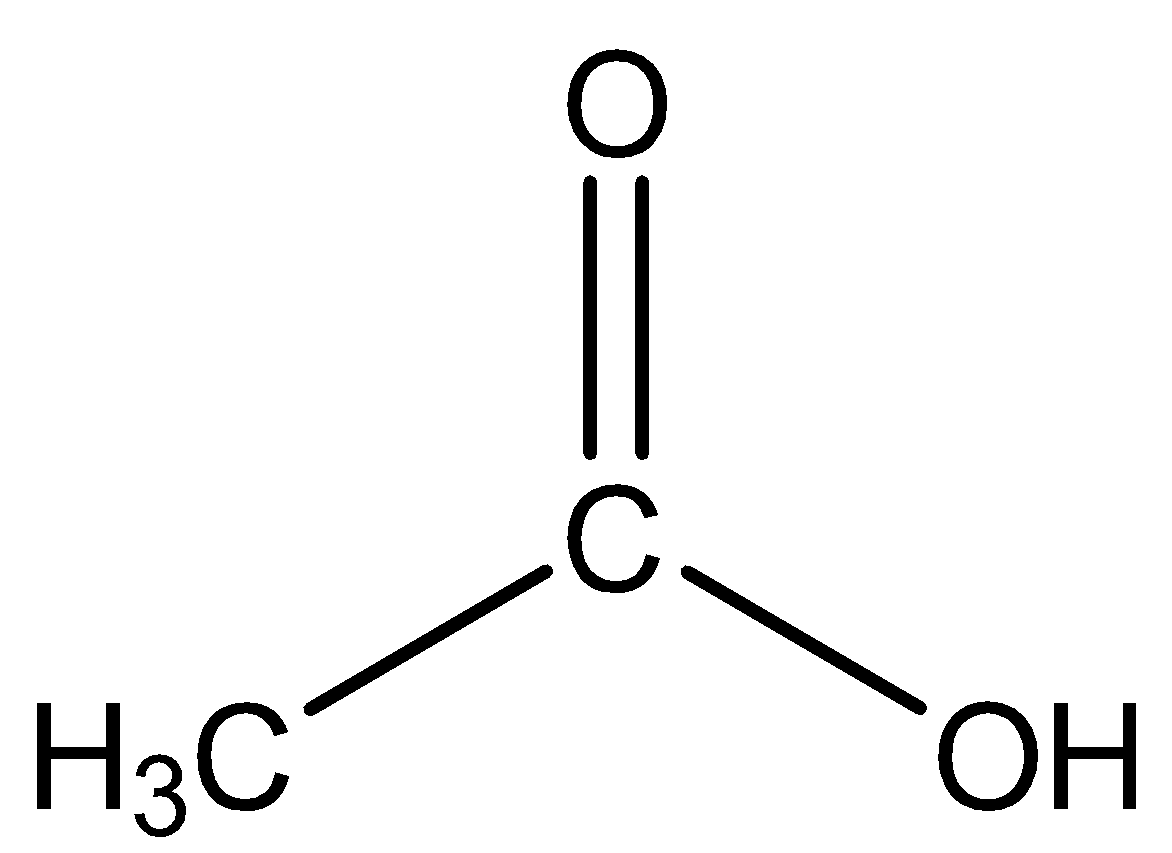
Here the methyl group attached is the electron donating group and thus will impose\[ + I\] effect thereby decreasing the acidic strength. Thus, acetic acid will be a weak acid.
(D) Formic acid: The structure is given below:
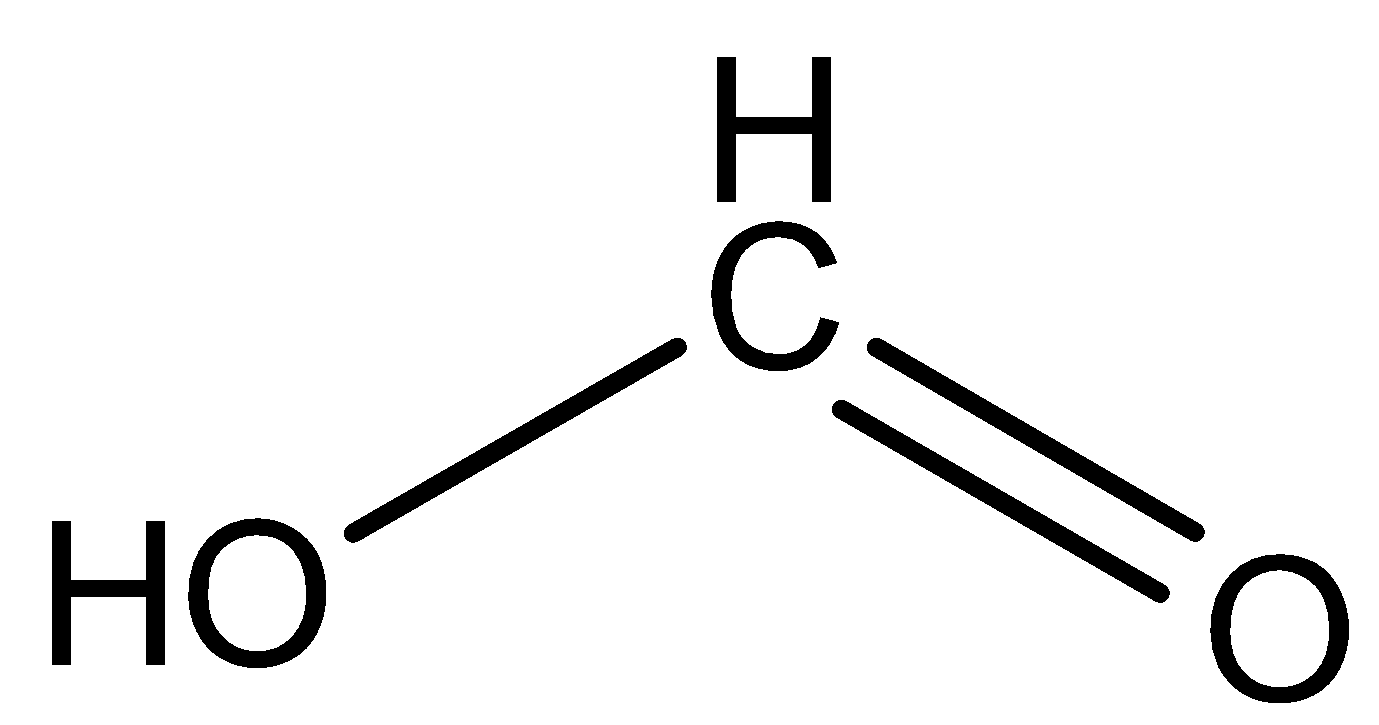
Here the hydrogen attached will impose none of the effect thereby does not either increase or decrease the acidic strength.
As a result, the correct order of decreasing acid strength will be\[B > A > D > C\].
So, the correct answer is Option C.
Note: There are certain factors that influence acidity which are: (i) Electronegativity: Acidity goes on increasing as you move from left to right in a periodic chart. (ii) Anion size: Acidity goes on increasing as you move from top to down in a periodic chart. (iii) Resonance: Anions having resonance structures are more acidic in comparison to anions which don't have.
Complete step by step answer:
In general, the acidic strength in an organic compound is directly related to the stability of the acid's conjugate base. In other words, an acid having a more stable conjugate base will be more acidic in comparison to an acid possessing a less stable conjugate base. In the given question, all four given acids are carboxylic acid ($ - COOH$) whose conjugate base ($ - CO{O^ - }$) includes a negative charge. This conjugate base can be stabilized if its carbon gets connected to an electron withdrawing group. Now let us look at the four given acids one by one:
(A) Trichloroacetic acid: The structure is given below:

Here three chloride groups attached are the electron withdrawing groups which impose the\[-I\] effect thereby increasing the acidic strength. Thus, trichloroacetic acid is highly acidic.
(B) Trifluoroacetic acid: The structure is given below:

Here three fluoride groups attached are the electron withdrawing groups which impose the\[-I\] effect thereby increasing the acidic strength. Thus, trifluoroacetic acid is highly acidic. Since\[-I\] effect of fluorine is more than that of chlorine, trifluoroacetic acid is more acidic in comparison to trichloroacetic acid.
(C) Acetic acid: The structure is given below:

Here the methyl group attached is the electron donating group and thus will impose\[ + I\] effect thereby decreasing the acidic strength. Thus, acetic acid will be a weak acid.
(D) Formic acid: The structure is given below:

Here the hydrogen attached will impose none of the effect thereby does not either increase or decrease the acidic strength.
As a result, the correct order of decreasing acid strength will be\[B > A > D > C\].
So, the correct answer is Option C.
Note: There are certain factors that influence acidity which are: (i) Electronegativity: Acidity goes on increasing as you move from left to right in a periodic chart. (ii) Anion size: Acidity goes on increasing as you move from top to down in a periodic chart. (iii) Resonance: Anions having resonance structures are more acidic in comparison to anions which don't have.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




