
The correct order of boiling point of the compounds is as follows:
A.
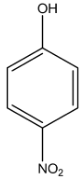
B.
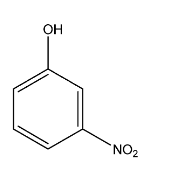
C.
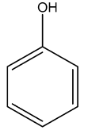
D.
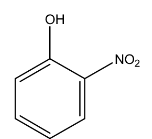
1.C > A > D > B
2.A > B > C > D
3.D > A > B > C
4.A > B > D > C




Answer
552.3k+ views
Hint:The boiling points of organic compounds are affected by different factors and among them the major ones include the intermolecular forces of attraction and the molecular weights of the compounds.
Complete step-by-step answer:Among the given compounds, there are three isomers of nitrophenol, the para-nitrophenol, the ortho-nitrophenol, and the meta-nitrophenol and only phenol. The boiling points of the isomers are affected by hydrogen bonding as the molecular weights of all are the same. The boiling point of para-nitrophenol is the highest followed by the meta-nitrophenol and finally the ortho-nitrophenol. This is due to the intermolecular hydrogen bonding in para-nitrophenol which is absent in both meta-nitrophenol and ortho-nitrophenol. This prevents the molecule from boiling faster.
The boiling point of phenol is lower than that of meta-nitrophenol and para-nitrophenol due to their higher molecular weight but it is higher than that of o-nitrophenol due to the presence of intermolecular hydrogen bonding among its molecules as well.
Hence, the correct order of bonding is as follows: A > B > C > D, and the correct answer is option 2.
Note: Phenol is a ortho, para orienting compound and when it is treated with a concentrated solution of nitric acid along with sulphuric acid, then it results in the formation of the para-nitrophenol and the ortho-nitrophenol. To get the meta-nitrophenol, the ortho and para positions need to be blocked first and then treated with the concentrated acid mixture.
Complete step-by-step answer:Among the given compounds, there are three isomers of nitrophenol, the para-nitrophenol, the ortho-nitrophenol, and the meta-nitrophenol and only phenol. The boiling points of the isomers are affected by hydrogen bonding as the molecular weights of all are the same. The boiling point of para-nitrophenol is the highest followed by the meta-nitrophenol and finally the ortho-nitrophenol. This is due to the intermolecular hydrogen bonding in para-nitrophenol which is absent in both meta-nitrophenol and ortho-nitrophenol. This prevents the molecule from boiling faster.
The boiling point of phenol is lower than that of meta-nitrophenol and para-nitrophenol due to their higher molecular weight but it is higher than that of o-nitrophenol due to the presence of intermolecular hydrogen bonding among its molecules as well.
Hence, the correct order of bonding is as follows: A > B > C > D, and the correct answer is option 2.
Note: Phenol is a ortho, para orienting compound and when it is treated with a concentrated solution of nitric acid along with sulphuric acid, then it results in the formation of the para-nitrophenol and the ortho-nitrophenol. To get the meta-nitrophenol, the ortho and para positions need to be blocked first and then treated with the concentrated acid mixture.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




