
The correct order of boiling point is:
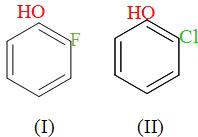
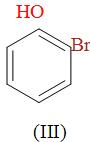
(a) I > II > III
(b) III > II > I
(c) II > I > III
(d) I > III > II


Answer
578.4k+ views
Hint: Boiling point in the o-substituted phenols is due to the intramolecular H-bonding and boiling point always increases with increase in the molecular weight of the substances and vice-versa. Now, identify the order of the boiling point.
Complete step by step answer:
By the term boiling point, we simply mean the temperature at which the particular substances begin to boil and get converted into its vapor phase.
The boiling point depends on the intermolecular (the bonding between the two molecules) and intramolecular (the bonding within the molecule) hydrogen bonding. Stronger the intramolecular bonding, greater is the boiling point and vice- versa. It is so because due to the H-bonding they hold a large no of the water molecules and require large energy to break such bonds.
Now, we have been given o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol i.e. belongs to the category of the phenols and their boiling point is determined on the basis of H-bonding.
In the above, we can see that all the substituents occupy the ortho -positions and in case of phenols, there is intramolecular H-bonding when the substituents are present at the ortho-positions. The substituents attached are the halogen atoms i.e. the fluorine, chlorine and bromine. Since, we know that all them i.e. o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol will have intramolecular H-bonding so there we can identify that who’s is having the higher boiling points through their molecular weights. It is so because the boiling point increases with the increase in the atomic weight.
So, among o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol, o-bromo-phenol has the highest boiling point and the o-fluoro-phenol has the lowest boiling point and o-chloro-phenol has the boiling point in between them because Br has the highest molecular weight thereafter followed by Cl and then the F.
So, the order of the boiling point is:
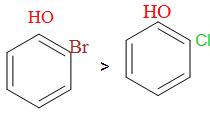
i.e. (III) > (II) > (I)
So, the correct answer is “Option B”.
Note: In ortho substituted phenols, due to close proximity of the phenol and the substituents, they form the intra =-molecular H-bonding and undergoes chelation and thus, therefore exist as discrete molecules (i.e. exist as separate molecules).
Complete step by step answer:
By the term boiling point, we simply mean the temperature at which the particular substances begin to boil and get converted into its vapor phase.
The boiling point depends on the intermolecular (the bonding between the two molecules) and intramolecular (the bonding within the molecule) hydrogen bonding. Stronger the intramolecular bonding, greater is the boiling point and vice- versa. It is so because due to the H-bonding they hold a large no of the water molecules and require large energy to break such bonds.
Now, we have been given o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol i.e. belongs to the category of the phenols and their boiling point is determined on the basis of H-bonding.
In the above, we can see that all the substituents occupy the ortho -positions and in case of phenols, there is intramolecular H-bonding when the substituents are present at the ortho-positions. The substituents attached are the halogen atoms i.e. the fluorine, chlorine and bromine. Since, we know that all them i.e. o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol will have intramolecular H-bonding so there we can identify that who’s is having the higher boiling points through their molecular weights. It is so because the boiling point increases with the increase in the atomic weight.
So, among o-fluoro-phenol, o-chloro-phenol and o-bromo-phenol, o-bromo-phenol has the highest boiling point and the o-fluoro-phenol has the lowest boiling point and o-chloro-phenol has the boiling point in between them because Br has the highest molecular weight thereafter followed by Cl and then the F.
So, the order of the boiling point is:


i.e. (III) > (II) > (I)
So, the correct answer is “Option B”.
Note: In ortho substituted phenols, due to close proximity of the phenol and the substituents, they form the intra =-molecular H-bonding and undergoes chelation and thus, therefore exist as discrete molecules (i.e. exist as separate molecules).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




