
The correct order of basic strength of the following compounds are
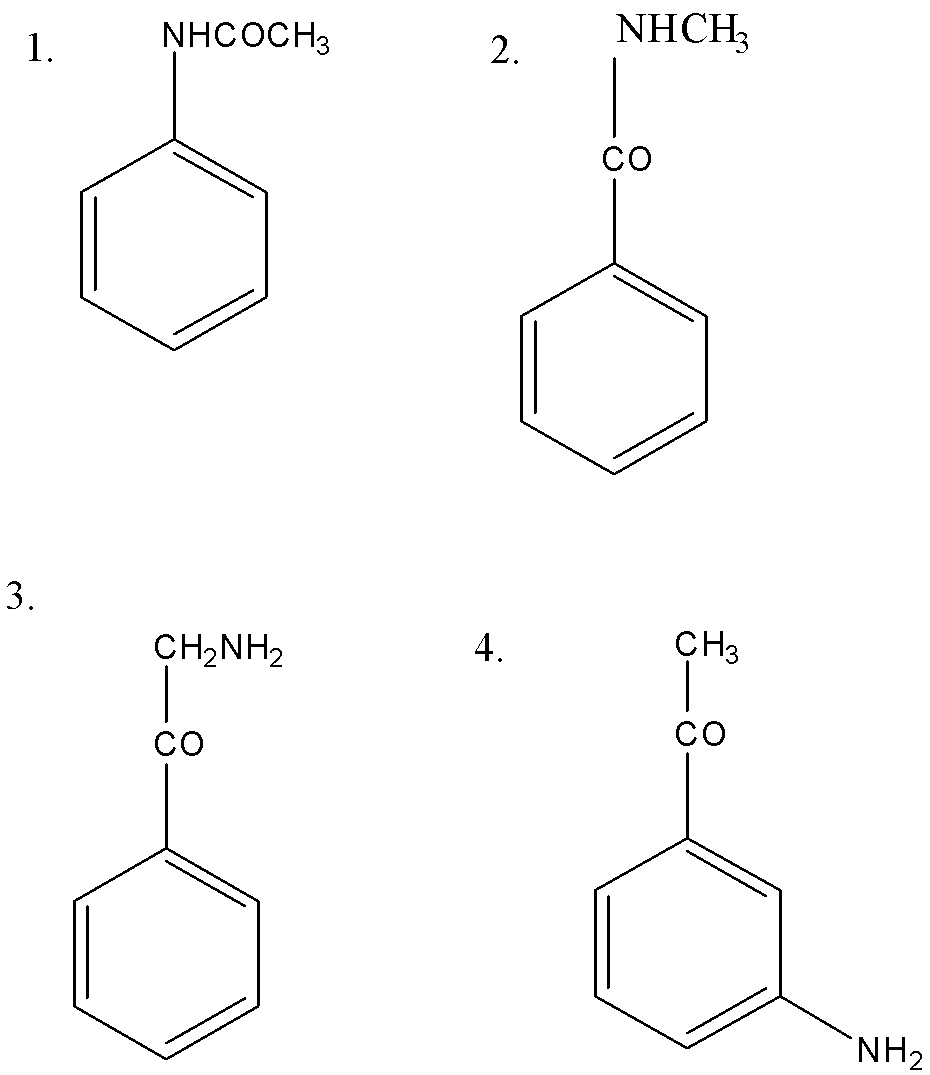
(A) $ 1 > 2 > 3 > 4 $
(B) $ 4 > 2 > 3 > 1 $
(C) $ 3 > 4 > 2 > 1 $
(D) $ 3 > 2 > 4 > 1 $

Answer
487.5k+ views
Hint: To determine the strength of a base, we look at the stability of the conjugate acid. Protonating the bottom nitrogen in the imidazole disrupts the aromaticity of the ring, because that nitrogen lone pair is part of the pi system. Resonance effects involving aromatic structures can have a dramatic influence on acidity and basicity.
Complete answer:
The basicity of an amine is increased by electron-donating groups and decreased by electron-withdrawing groups. Aryl amines are less basic than alkyl-substituted amines because some electron density provided by the nitrogen atom is distributed throughout the aromatic ring. An electron releasing group increases the electron density on the $ N - $ atom.
compound $ (1) $ is the beast basic among all. In compound $ (2) $ , $ - NH - $ is attached with one electron withdrawing group $ - CO - $ and one electron releasing group $ - C{H_3} $ , it is a bit more basic than compound $ (1) $ . In compound $ (4) $ $ - COC{H_3} $ group is attached to aniline ring, not directly with $ - N{H_2} $ group. Hence it is more basic than compound $ (2) $ . Compound $ (3) $ is the most basic among all compounds since in it, $ - N{H_2} $ is attached to only one electron releasing group.
Therefore, the option (C) $ 3 > 4 > 2 > 1 $ is correct.
Note:
The basicity of heterocyclic amines depends on the location of the electron pair of the nitrogen atom, its hybridization, and whether or not resonance stabilization is possible. In pyrrole, the electron pair is part of the aromatic system. As a result, pyrrole is a very weak base. Pyridine is a weaker base than saturated amines of similar structure because its electron pair is in an $ s{p^2} $ -hybridized orbital, and the electron pair is more tightly held by the atom.
Complete answer:
The basicity of an amine is increased by electron-donating groups and decreased by electron-withdrawing groups. Aryl amines are less basic than alkyl-substituted amines because some electron density provided by the nitrogen atom is distributed throughout the aromatic ring. An electron releasing group increases the electron density on the $ N - $ atom.
compound $ (1) $ is the beast basic among all. In compound $ (2) $ , $ - NH - $ is attached with one electron withdrawing group $ - CO - $ and one electron releasing group $ - C{H_3} $ , it is a bit more basic than compound $ (1) $ . In compound $ (4) $ $ - COC{H_3} $ group is attached to aniline ring, not directly with $ - N{H_2} $ group. Hence it is more basic than compound $ (2) $ . Compound $ (3) $ is the most basic among all compounds since in it, $ - N{H_2} $ is attached to only one electron releasing group.
Therefore, the option (C) $ 3 > 4 > 2 > 1 $ is correct.
Note:
The basicity of heterocyclic amines depends on the location of the electron pair of the nitrogen atom, its hybridization, and whether or not resonance stabilization is possible. In pyrrole, the electron pair is part of the aromatic system. As a result, pyrrole is a very weak base. Pyridine is a weaker base than saturated amines of similar structure because its electron pair is in an $ s{p^2} $ -hybridized orbital, and the electron pair is more tightly held by the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




