
The correct order of acidic strength is:
A.$C{H_3}COOH < HCN < {H_2}O < {C_2}{H_5}OH$
B.$C{H_3}COOH > HCN > {H_2}O > {C_2}{H_5}OH$
C.$HCN > \,C{H_3}COOH > \,{H_2}O > \,{C_2}{H_5}OH$
D.$C{H_3}COOH > HCN > {C_2}{H_5}OH > {H_2}O$
Answer
559.2k+ views
Hint:: For solving questions of acidic strength and basic strength we have to firstly make their conjugate base and conjugate acid. You must be familiar with Bronsted Lowry theory in which we have to make conjugate acid and base. The conjugate acid base pair is more stable than the corresponding acid or base should be strong.
Complete step-by-step answer:According to Bronsted Lowry theory, acids are good proton donor then after donating a proton they will form conjugate base while on the other hand we have bases which are good proton acceptor thus after accepting one proton they will form conjugate acid. These conjugate acid or bases are stable means that corresponding bases and acid are strong. Let’s consider acetic acid and hydrogen cyanide both are weak acids but among them when proton is released and corresponding conjugate bases are formed. The conjugate base of acetic acid is more stable thus it is weak and acetic acid is strong this is due to the delocalization of negative charge.
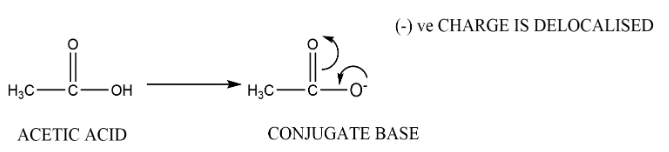
Similarly when we talk about alcohol and water, among them water is more acidic. This can be explained when we take out one proton from each and make their conjugate bases, the conjugate base of ethanol is ethoxide ion while for water the conjugate base is hydroxide ion. The ethoxide ion is a stronger base than hydroxide ions. Thus this makes water more acidic than ethanol.
The order will be $C{H_3}COOH > HCN > {H_2}O > {C_2}{H_5}OH$
Option B is correct.
Note: The same order can be written when we consider phenol, alcohol water etc. The same process of making conjugate base first and then its stability of conjugate base is seen. There is a relation between acidic strength and $p{K_a}$ value, where they are inversely proportional so if we have to write the order of $p{K_a}$ then we just have to reverse the sign.
Complete step-by-step answer:According to Bronsted Lowry theory, acids are good proton donor then after donating a proton they will form conjugate base while on the other hand we have bases which are good proton acceptor thus after accepting one proton they will form conjugate acid. These conjugate acid or bases are stable means that corresponding bases and acid are strong. Let’s consider acetic acid and hydrogen cyanide both are weak acids but among them when proton is released and corresponding conjugate bases are formed. The conjugate base of acetic acid is more stable thus it is weak and acetic acid is strong this is due to the delocalization of negative charge.

Similarly when we talk about alcohol and water, among them water is more acidic. This can be explained when we take out one proton from each and make their conjugate bases, the conjugate base of ethanol is ethoxide ion while for water the conjugate base is hydroxide ion. The ethoxide ion is a stronger base than hydroxide ions. Thus this makes water more acidic than ethanol.
The order will be $C{H_3}COOH > HCN > {H_2}O > {C_2}{H_5}OH$
Option B is correct.
Note: The same order can be written when we consider phenol, alcohol water etc. The same process of making conjugate base first and then its stability of conjugate base is seen. There is a relation between acidic strength and $p{K_a}$ value, where they are inversely proportional so if we have to write the order of $p{K_a}$ then we just have to reverse the sign.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




