
The correct geometry and hybridization for \[Xe{{F}_{4}}\] are:
A. Trigonal planar, \[s{{p}^{3}}{{d}^{3}}\]
B. Square planar, \[s{{p}^{3}}{{d}^{2}}\]
C. Octahedral, \[s{{p}^{3}}{{d}^{2}}\]
D. Trigonal bipyramidal, \[s{{p}^{3}}d\]
Answer
603.3k+ views
Hint: By using VSEPR theory (Valence Shell Electron Pair Repulsion theory) we can find the structure and hybridization of the molecules. It is also named as “Gillespie-Nyholm theory” after its two main developers, Ronald Gillespie and Ronald Nyholm.
Complete step by step answer:
Xenon is a noble gas that has 8 valence electrons.
There are 4 Fluorine atoms attached or bonded with Xe, each of a single bond.
We will get a number of lone pair electrons on Xenon after subtracting the bonding electrons from the total number of atoms in the valence shell of the xenon.
So, the number of lone pair or non-bonding electrons surrounding of xenon atom is
8–4=4 (that means: two lone pair electrons).
Therefore, \[Xe{{F}_{4}}\] molecule has 4 bond pairs and 2 lone pairs in the molecule.
We will get lone pair of electrons by adding the electron pairs surrounding the xenon atom,
4+2=6, where 6 signifies octahedral geometry.
So, the geometry of the molecule is octahedral \[s{{p}^{3}}{{d}^{2}}\] hybridization.
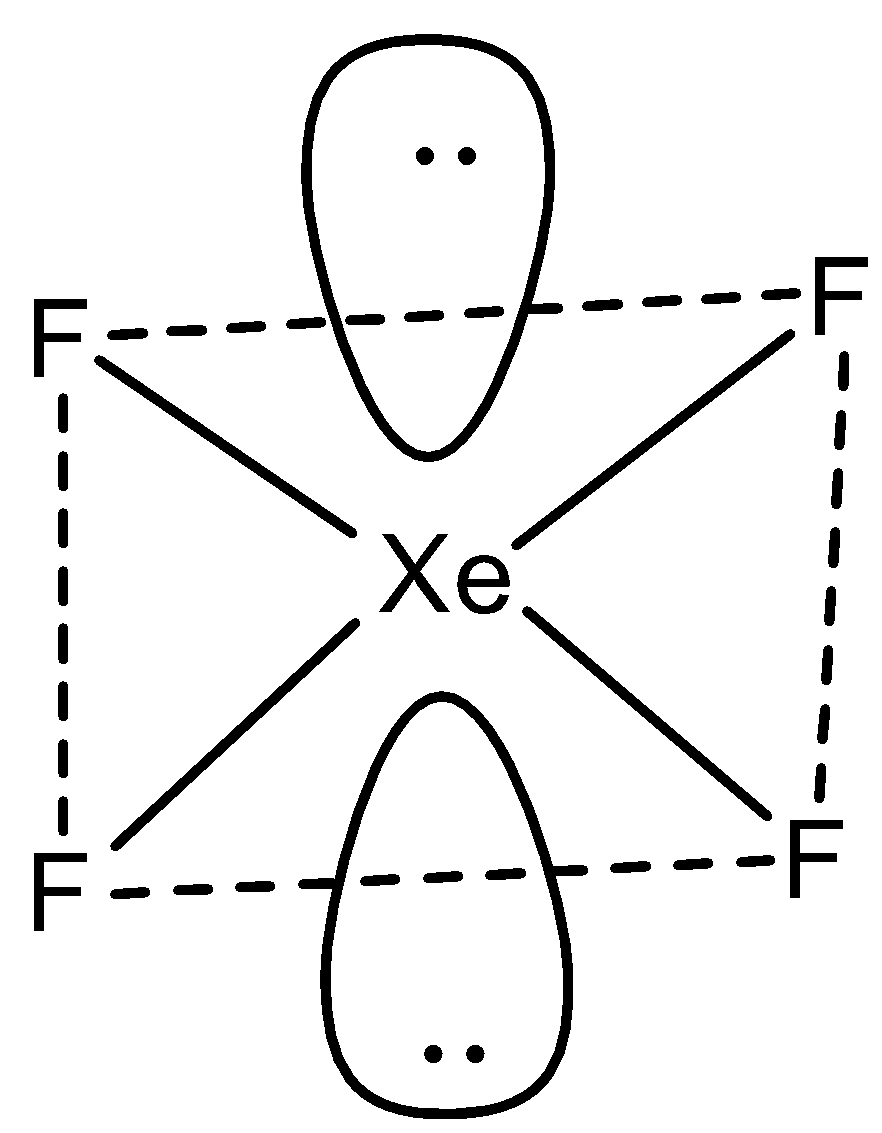
But in the structure all fluorine atoms are in one plane and lone pairs of electrons in another plane.
So, from the structure it is clear that the shape of the molecule (\[Xe{{F}_{4}}\]) is Square planar and hybridization is\[s{{p}^{3}}{{d}^{2}}\].
So, the correct option is B.
Note: Don’t be confused with the words, octahedral and tetrahedral.
According to VSEPR theory the octahedral shaped molecule should have 6 bond pairs, but the molecule \[Xe{{F}_{4}}\] has four bond pairs and 2 lone pairs. So, the molecule contains four bond pairs and two lone pairs of electrons in the structure, then the shape of the molecule is square planar.
Complete step by step answer:
Xenon is a noble gas that has 8 valence electrons.
There are 4 Fluorine atoms attached or bonded with Xe, each of a single bond.
We will get a number of lone pair electrons on Xenon after subtracting the bonding electrons from the total number of atoms in the valence shell of the xenon.
So, the number of lone pair or non-bonding electrons surrounding of xenon atom is
8–4=4 (that means: two lone pair electrons).
Therefore, \[Xe{{F}_{4}}\] molecule has 4 bond pairs and 2 lone pairs in the molecule.
We will get lone pair of electrons by adding the electron pairs surrounding the xenon atom,
4+2=6, where 6 signifies octahedral geometry.
So, the geometry of the molecule is octahedral \[s{{p}^{3}}{{d}^{2}}\] hybridization.

But in the structure all fluorine atoms are in one plane and lone pairs of electrons in another plane.
So, from the structure it is clear that the shape of the molecule (\[Xe{{F}_{4}}\]) is Square planar and hybridization is\[s{{p}^{3}}{{d}^{2}}\].
So, the correct option is B.
Note: Don’t be confused with the words, octahedral and tetrahedral.
According to VSEPR theory the octahedral shaped molecule should have 6 bond pairs, but the molecule \[Xe{{F}_{4}}\] has four bond pairs and 2 lone pairs. So, the molecule contains four bond pairs and two lone pairs of electrons in the structure, then the shape of the molecule is square planar.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




