
The correct decreasing order of nearest bond angle:
\[
A:CI{F_3} > P{F_3} > N{F_3} > B{F_3} \\
B:B{F_3} > P{F_3} > N{F_3} > CI{F_3} \\
C:B{F_3} > CI{F_3} > P{F_3} > N{F_3} \\
D:B{F_3} > N{F_3} > P{F_3} > CI{F_3} \\
\]
Answer
569.7k+ views
Hint: A bond angle refers to the angle between the two bonds which originate from the same atom in any covalent species. For example, let us consider a molecule ABC in which B and C atoms are bonded to the atom A then, the angle existing between the lines AB and AC is referred to as the bond angle.
Complete Step by step answer:
Bond angles contribute to the shape of the molecules. The bond angle also helps to differentiate between the linear, trigonal planar, trigonal-bipyramidal, tetrahedral and octahedral geometries of the molecule.
To compare the bond angles, keep certain points in mind:
-Lone pair of electrons on the central atom tries to repel the bonded pair of electrons. Thus, bonds are displaced slightly which results in a decrease of the bond angle with more lone pairs of electrons.
-Bond angle also decreases if the electronegativity of the central atom is decreased.
In the present question, please refer to the following structure of the compounds mentioned
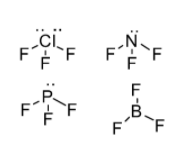
According to the structures drawn consider the following facts about these compounds:
-\[Cl{F_3}\]comprises of two lone pair of electrons thus, its bond angle will be the least
-\[B{F_3}\]possess no lone pair of electrons thus, it has the maximum bond angle
-Bond angle of \[P{F_3}\]is greater than \[N{F_3}\]because of more repulsion between \[P - F\] bonds due to the resonance effect.
Hence, the correct decreasing order of nearest bond angle is Option B i.e. \[B{F_3} > P{F_3} > N{F_3} > CI{F_3}\]
Note: Always remember that the stronger the bond is, shorter will be the bond length. This means that single bonds are always longer than the double bonds which are again longer than the triple bonds. The order of bond length is triple bond < double bond < single bond.
Complete Step by step answer:
Bond angles contribute to the shape of the molecules. The bond angle also helps to differentiate between the linear, trigonal planar, trigonal-bipyramidal, tetrahedral and octahedral geometries of the molecule.
To compare the bond angles, keep certain points in mind:
-Lone pair of electrons on the central atom tries to repel the bonded pair of electrons. Thus, bonds are displaced slightly which results in a decrease of the bond angle with more lone pairs of electrons.
-Bond angle also decreases if the electronegativity of the central atom is decreased.
In the present question, please refer to the following structure of the compounds mentioned

According to the structures drawn consider the following facts about these compounds:
-\[Cl{F_3}\]comprises of two lone pair of electrons thus, its bond angle will be the least
-\[B{F_3}\]possess no lone pair of electrons thus, it has the maximum bond angle
-Bond angle of \[P{F_3}\]is greater than \[N{F_3}\]because of more repulsion between \[P - F\] bonds due to the resonance effect.
Hence, the correct decreasing order of nearest bond angle is Option B i.e. \[B{F_3} > P{F_3} > N{F_3} > CI{F_3}\]
Note: Always remember that the stronger the bond is, shorter will be the bond length. This means that single bonds are always longer than the double bonds which are again longer than the triple bonds. The order of bond length is triple bond < double bond < single bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




