
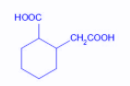
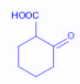
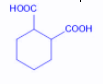
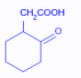
The compound that undergoes decarboxylation most readily under mild conditions is:
A.
B.
C.
D.




Answer
573.3k+ views
Hint: To answer this question, you must recall the process and mechanism of decarboxylation. Decarboxylation of an acid is the removal of the carboxylic group of the acid in the form of carbon dioxide. $\beta $ -keto acids undergo decarboxylation reaction easily.
Complete step by step solution:
A decarboxylation reaction can be most simply termed as a reaction where there is a loss of carbon dioxide. The general decarboxylation reaction can be written as:
$RCOOH \to R - H + C{O_2}$
Simple carboxylic acids do not undergo a decarboxylation reaction easily. Whereas, carboxylic acids having a carbonyl group attached at the second carbon or the $\beta $ carbon atom, that is, $\beta $ -keto acids undergo the loss of carbon dioxide very easily. Since simple carboxylic acids are stable, they undergo decarboxylation only under harsh conditions unlike $\beta $ -keto acids which easily undergo the reaction at mild conditions as well.
Thus, we search for the beta keto acid out of the given options. The compound in option B is a $\beta $ -keto acid and thus can undergo decarboxylation reaction under mild conditions.
Hence, the correct option is B.
Note:
$\beta $ -keto acids are unstable because the ketone group present at the $\beta $ position accepts a hydrogen ion from the acidic carboxylic group forming carboxylate group and a carbocation at the $\beta $ carbon atom. A six membered cyclic transition state is formed and this anion is highly unstable. Thus, the carboxylic group shifts its electron density towards the positively charged carbon atom and leaves the compound as carbon dioxide gas. As a result, an enol is formed which tautomerizes to form a ketone.
Complete step by step solution:
A decarboxylation reaction can be most simply termed as a reaction where there is a loss of carbon dioxide. The general decarboxylation reaction can be written as:
$RCOOH \to R - H + C{O_2}$
Simple carboxylic acids do not undergo a decarboxylation reaction easily. Whereas, carboxylic acids having a carbonyl group attached at the second carbon or the $\beta $ carbon atom, that is, $\beta $ -keto acids undergo the loss of carbon dioxide very easily. Since simple carboxylic acids are stable, they undergo decarboxylation only under harsh conditions unlike $\beta $ -keto acids which easily undergo the reaction at mild conditions as well.
Thus, we search for the beta keto acid out of the given options. The compound in option B is a $\beta $ -keto acid and thus can undergo decarboxylation reaction under mild conditions.
Hence, the correct option is B.
Note:
$\beta $ -keto acids are unstable because the ketone group present at the $\beta $ position accepts a hydrogen ion from the acidic carboxylic group forming carboxylate group and a carbocation at the $\beta $ carbon atom. A six membered cyclic transition state is formed and this anion is highly unstable. Thus, the carboxylic group shifts its electron density towards the positively charged carbon atom and leaves the compound as carbon dioxide gas. As a result, an enol is formed which tautomerizes to form a ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




