
The compound that is most reactive towards electrophilic nitration is:
A. Toluene
B. Benzene
C. Benzoic acid
D. Nitrobenzene
Answer
569.1k+ views
Hint: We know that nitration is a reaction in which a nitro group displaces a hydrogen atom in a compound. A nitro group is an electron withdrawing group and nitration happens to that compound if the compound has an electron releasing group.
Complete step by step answer:
Let’s discuss the possibility of electrophilic nitration in all cases.
Option A is toluene. The structure of toluene is,
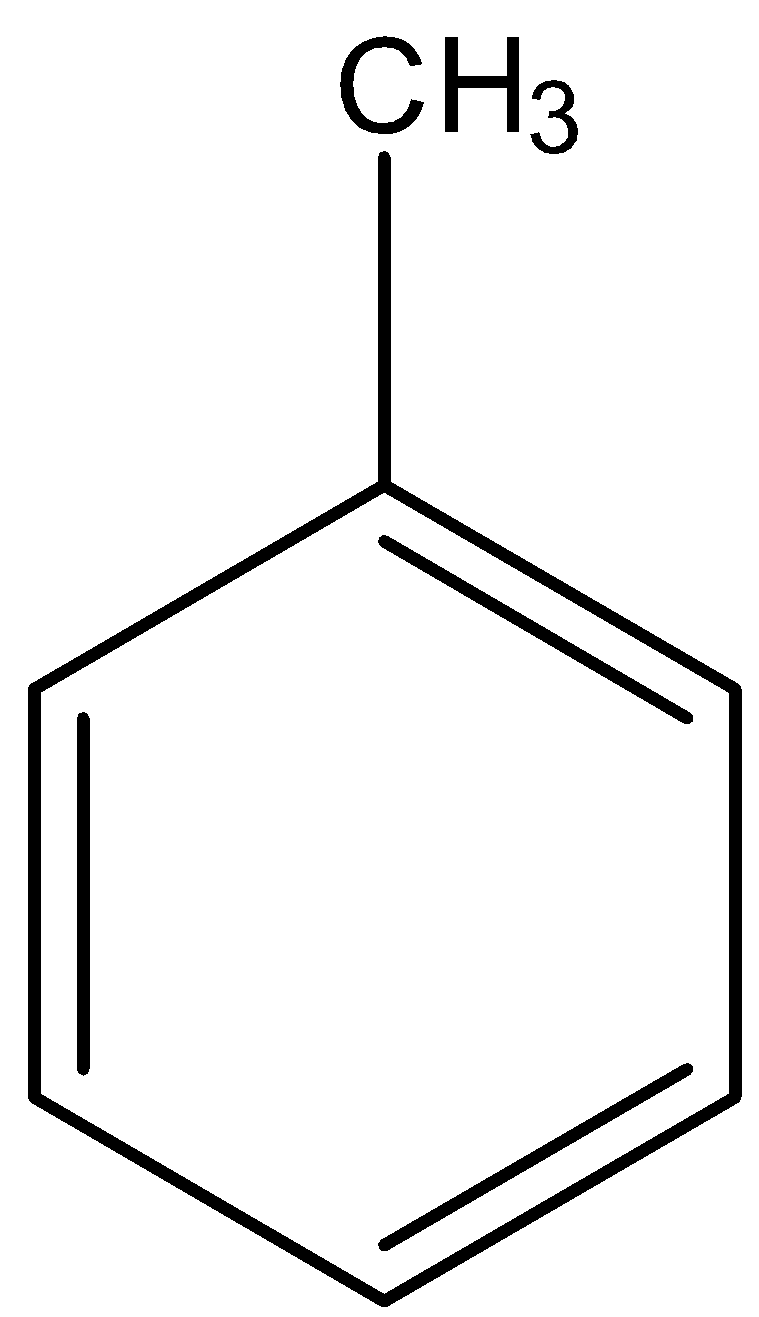
We know that the $CH_3$ group is an electron releasing group. And we know that the nitro group is an electron withdrawing group. So, the electrophilic substitution of the $NO_2$ group is possible here.
Option B is benzene. The structure of benzene is,
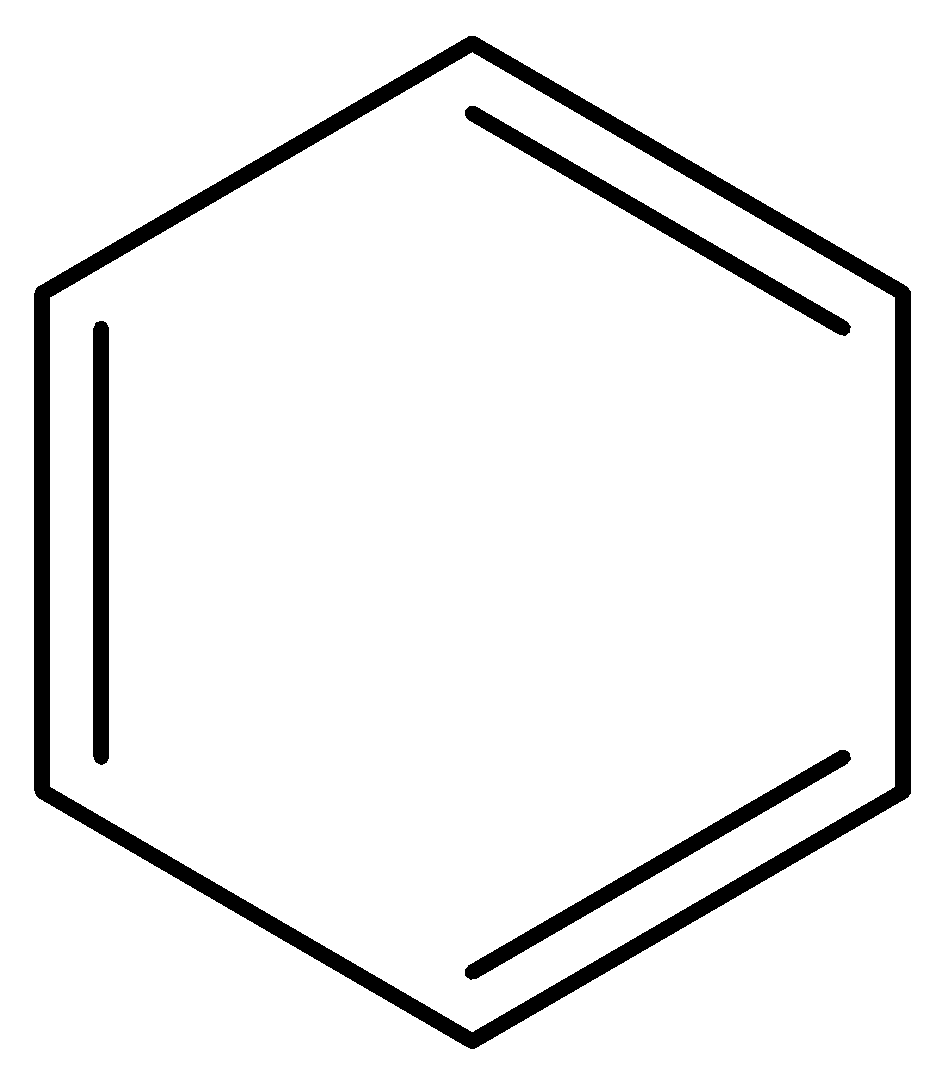
Here, also nitration is possible. But its reactivity towards electrophilic nitration is less than toluene because of absence of electron releasing group. Option C is benzoic acid. The structure of benzoic acid is,
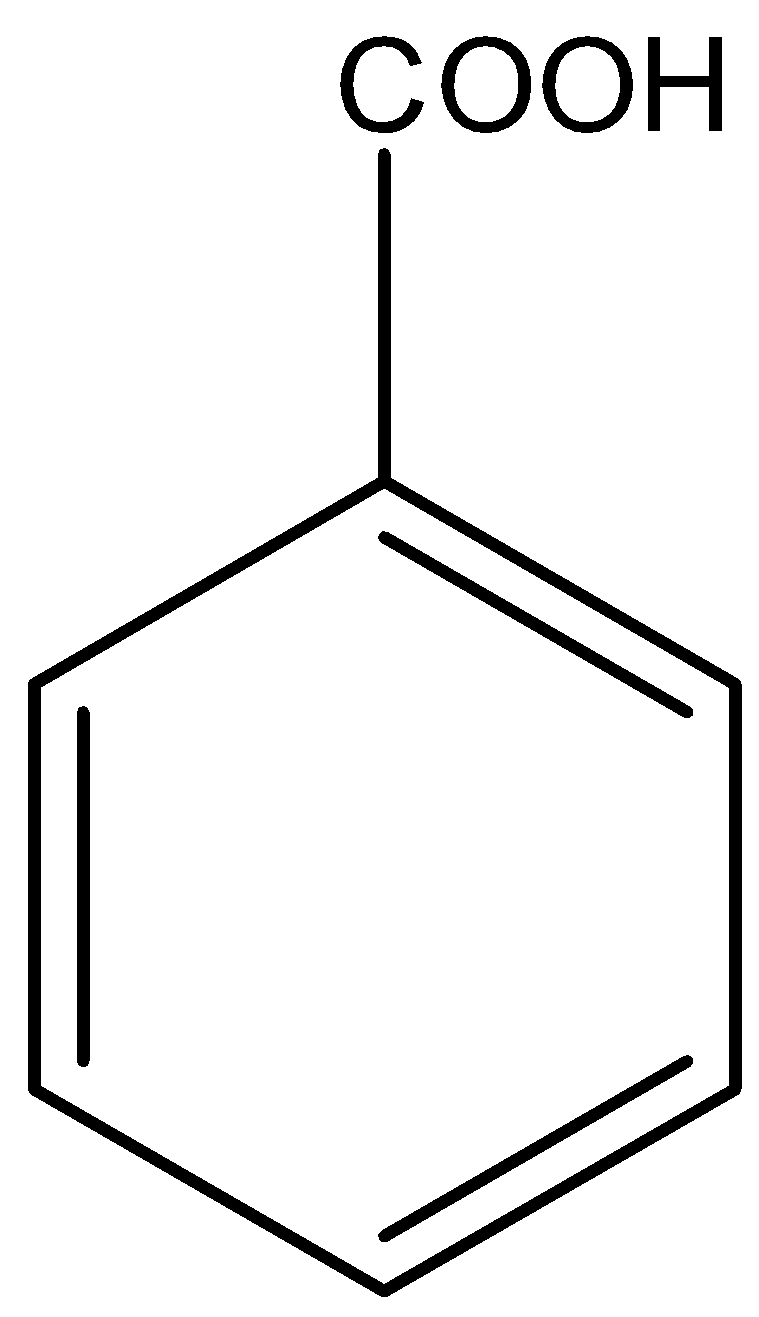
Here, COOH group is an electron withdrawing group. So, electrophilic nitration is less reactive in benzoic acid than benzene as the nitro group is also an electron withdrawing group.
Option D is nitrobenzene. The structure of nitrobenzene is,
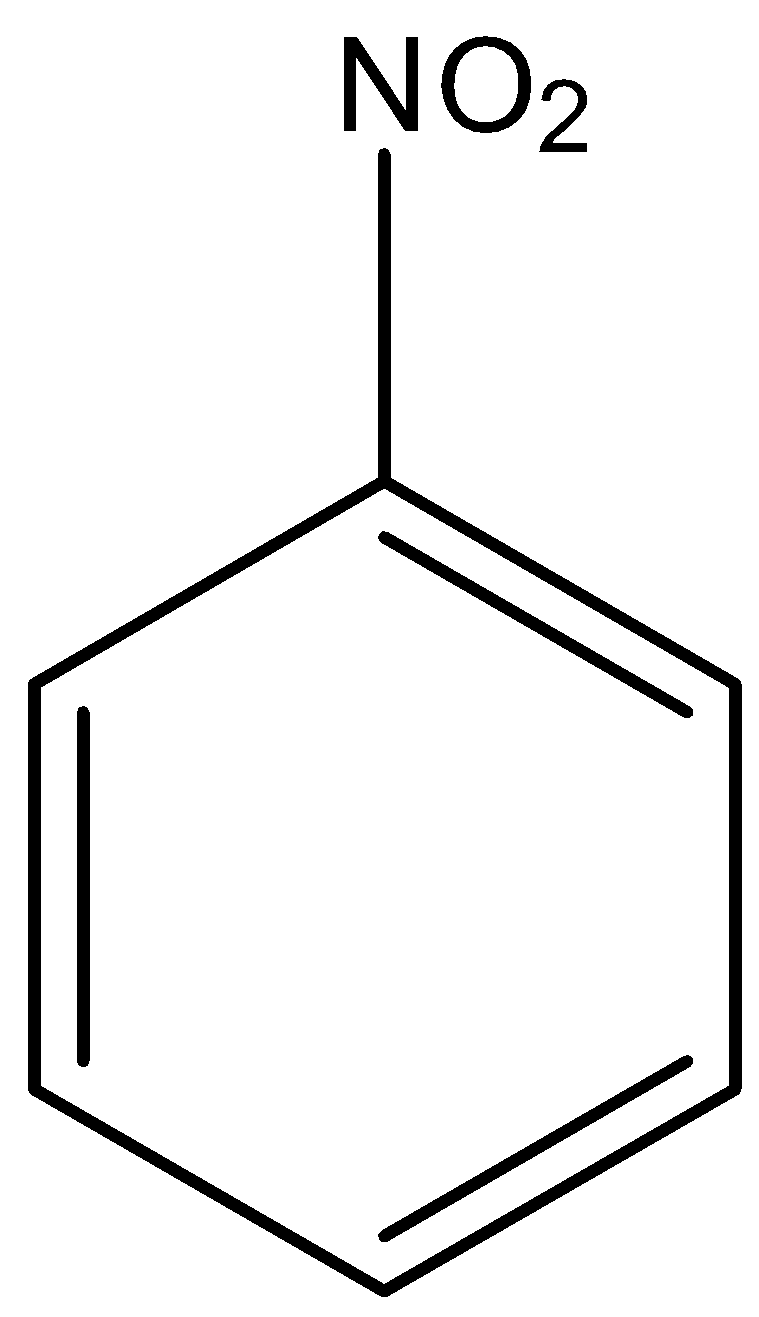
We know that the nitro group is an electron withdrawing group. So, electrophilic nitration is less reactive than benzene.
So, the compound which is more reactive towards electrophilic nitration is toluene.
So, the correct answer is Option A.
Note: Always remember that substituents having pi bonds to electronegative atoms (C=O, -$NO_2$) adjacent to the pi system are electron withdrawing groups. They deactivate the aromatic ring by decreasing electron density on the ring by resonance.
Complete step by step answer:
Let’s discuss the possibility of electrophilic nitration in all cases.
Option A is toluene. The structure of toluene is,

We know that the $CH_3$ group is an electron releasing group. And we know that the nitro group is an electron withdrawing group. So, the electrophilic substitution of the $NO_2$ group is possible here.
Option B is benzene. The structure of benzene is,

Here, also nitration is possible. But its reactivity towards electrophilic nitration is less than toluene because of absence of electron releasing group. Option C is benzoic acid. The structure of benzoic acid is,

Here, COOH group is an electron withdrawing group. So, electrophilic nitration is less reactive in benzoic acid than benzene as the nitro group is also an electron withdrawing group.
Option D is nitrobenzene. The structure of nitrobenzene is,

We know that the nitro group is an electron withdrawing group. So, electrophilic nitration is less reactive than benzene.
So, the compound which is more reactive towards electrophilic nitration is toluene.
So, the correct answer is Option A.
Note: Always remember that substituents having pi bonds to electronegative atoms (C=O, -$NO_2$) adjacent to the pi system are electron withdrawing groups. They deactivate the aromatic ring by decreasing electron density on the ring by resonance.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




