
The compound having only primary hydrogen atoms is:
A. isobutene
B. \[2,3 - \]Dimethyl\[ - 1 - \]butene
C. cyclohexane
D. propane
Answer
567.9k+ views
Hint: To answer this question we should know what primary hydrogen is. The hydrogen attached with primary carbon is known as primary hydrogen. We will draw the geometry of all the given molecules then we will find the primary carbon to determine the compound having all primary hydrogen atoms.
Complete step-by-step answer:
A carbon atom has four valencies. Primary carbon is the carbon which is attached with one other carbon only other three valencies are satisfied by hydrogen atoms.
The primary carbon is shown as follows:
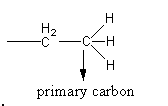
The hydrogen atoms attached with primary carbon are known as primary hydrogen atoms which are shown as follows:
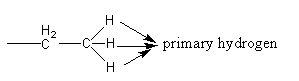
The structure of the given compounds are as follows:
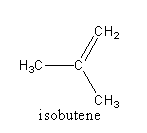
In isobutene, three carbon are primary because they are attached with only one carbon atom. So, all hydrogen is primary.
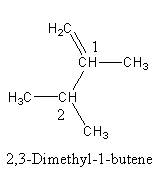
In \[2,3 - \]Dimethyl\[ - 1 - \]butene, four carbon are primary except the carbon indicated by $1$ and $2$. The carbon indicated by $1$ and $2$ also has one hydrogen atom.
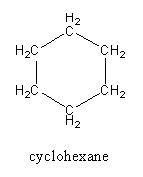
In cyclohexane, every carbon is attached with two other carbon atoms, so cyclohexane does not have any primary carbon hence no primary hydrogen atoms.
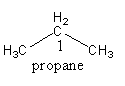
In propane, two primary carbon are present except the carbon indicated by $1$. The carbon indicated by $1$ also has two hydrogen atoms.
So, the compound having only primary hydrogen atoms is isobutene.
Therefore, option (A) isobutene, is correct.
Note: The carbon attached with two other carbon is known as secondary carbon and the hydrogen atoms attached with secondary carbon are known as secondary hydrogen atoms. The carbon attached with three other carbon is known as tertiary carbon and the hydrogen atoms attached with tertiary carbon are known as tertiary hydrogen atoms. Here, except cyclohexane, all have primary carbon and primary hydrogen but only isobutene has all primary hydrogen. Isobutene has tertiary carbon but no the tertiary hydrogen.
Complete step-by-step answer:
A carbon atom has four valencies. Primary carbon is the carbon which is attached with one other carbon only other three valencies are satisfied by hydrogen atoms.
The primary carbon is shown as follows:

The hydrogen atoms attached with primary carbon are known as primary hydrogen atoms which are shown as follows:

The structure of the given compounds are as follows:

In isobutene, three carbon are primary because they are attached with only one carbon atom. So, all hydrogen is primary.

In \[2,3 - \]Dimethyl\[ - 1 - \]butene, four carbon are primary except the carbon indicated by $1$ and $2$. The carbon indicated by $1$ and $2$ also has one hydrogen atom.

In cyclohexane, every carbon is attached with two other carbon atoms, so cyclohexane does not have any primary carbon hence no primary hydrogen atoms.

In propane, two primary carbon are present except the carbon indicated by $1$. The carbon indicated by $1$ also has two hydrogen atoms.
So, the compound having only primary hydrogen atoms is isobutene.
Therefore, option (A) isobutene, is correct.
Note: The carbon attached with two other carbon is known as secondary carbon and the hydrogen atoms attached with secondary carbon are known as secondary hydrogen atoms. The carbon attached with three other carbon is known as tertiary carbon and the hydrogen atoms attached with tertiary carbon are known as tertiary hydrogen atoms. Here, except cyclohexane, all have primary carbon and primary hydrogen but only isobutene has all primary hydrogen. Isobutene has tertiary carbon but no the tertiary hydrogen.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




