
The compound formed as a result of oxidation of ethylbenzene by \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is:
A.benzophenone
B.acetophenone
C.benzoic acid
D.benzyl alcohol
Answer
570k+ views
Hint: Ethyl benzene is a cyclic compound. On undergoing oxidation in the presence of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\], the product will be also a cyclic compound.
Complete step by step solution:
We know that the formula of ethyl benzene is \[{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}\]. We will see what this compound will give in the presence of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\].
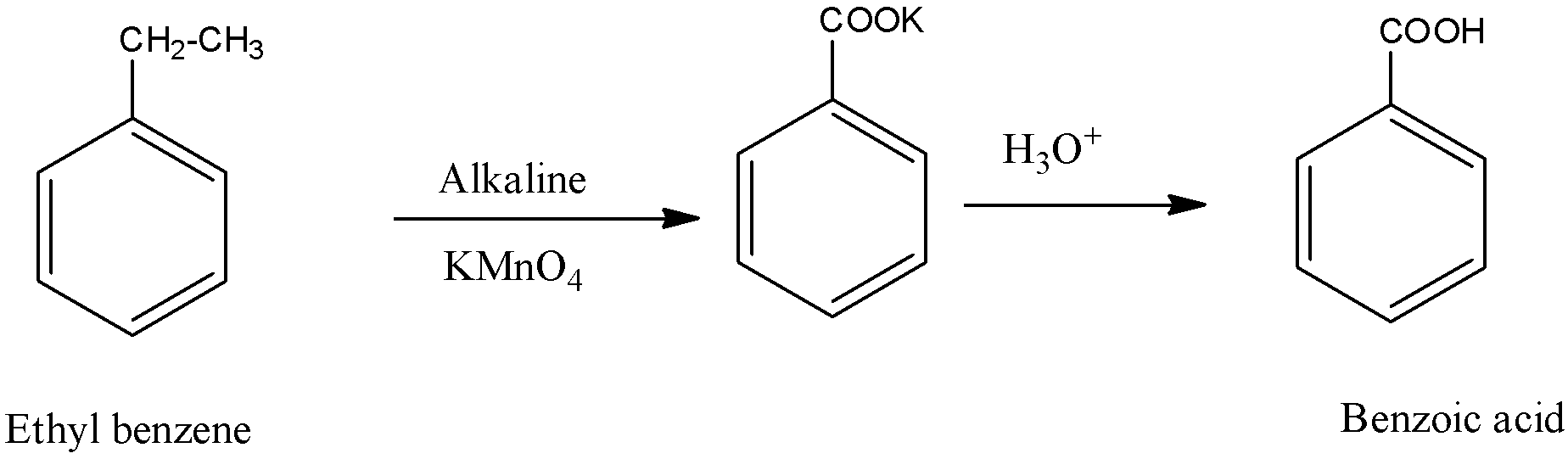
In the above reaction, we see that ethyl alcohol gives benzoic acid during the oxidation of ethyl benzene by \[{\text{KMn}}{{\text{O}}_{\text{4}}}\].
Therefore,C is the correct option.
Additional information:
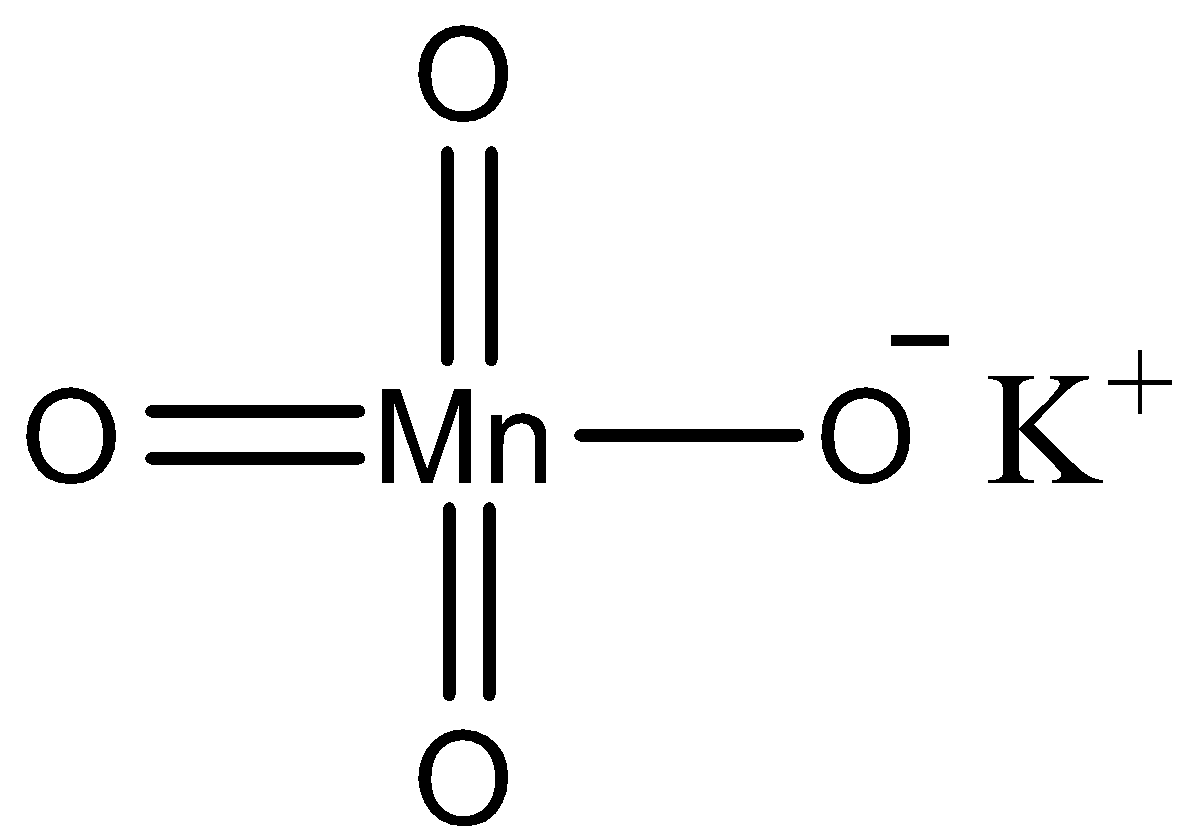
\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is a very common reagent used in the oxidation of various compounds. We can draw the structure of potassium permanganate as follows.
\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is an odourless, purple coloured crystalline solid. It is soluble in water, acetic acid, acetone, methanol, pyridine etc. It is soluble in solvents like ethanol and in other organic solvents. Potassium permanganate exists in the form of monoclinic prisms. \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is opaque with a blue metallic lustre. Potassium permanganate is a strong oxidizing agent, and it is used as an oxidant in a lot of chemical reactions.
When potassium permanganate in solid form is heated, it undergoes decomposition. The reaction is as follows:
\[{\mathbf{2KMn}}{{\mathbf{O}}_{\mathbf{4}}}\; \to {\text{ }}{{\mathbf{K}}_{\mathbf{2}}}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{2}}}\left( {\mathbf{s}} \right){\text{ }} + {\text{ }}{{\mathbf{O}}_{\mathbf{2}}}\]
On heating with an alkali, potassium permanganate it produces manganese and oxygen gas is evolved.
\[{\mathbf{4KMn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{4KOH}}\; \to {\text{ }}{\mathbf{4}}{{\mathbf{K}}_{\mathbf{2}}}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{2}}{{\mathbf{H}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} + {\text{ }}{{\mathbf{O}}_{\mathbf{2}}}\]
Note: In qualitative analysis, Potassium permanganate is used for determining the permanganate value. It is also used in the treatment of various bacterial infections.
Complete step by step solution:
We know that the formula of ethyl benzene is \[{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}\]. We will see what this compound will give in the presence of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\].

In the above reaction, we see that ethyl alcohol gives benzoic acid during the oxidation of ethyl benzene by \[{\text{KMn}}{{\text{O}}_{\text{4}}}\].
Therefore,C is the correct option.
Additional information:
\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is a very common reagent used in the oxidation of various compounds. We can draw the structure of potassium permanganate as follows.

\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is an odourless, purple coloured crystalline solid. It is soluble in water, acetic acid, acetone, methanol, pyridine etc. It is soluble in solvents like ethanol and in other organic solvents. Potassium permanganate exists in the form of monoclinic prisms. \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is opaque with a blue metallic lustre. Potassium permanganate is a strong oxidizing agent, and it is used as an oxidant in a lot of chemical reactions.
When potassium permanganate in solid form is heated, it undergoes decomposition. The reaction is as follows:
\[{\mathbf{2KMn}}{{\mathbf{O}}_{\mathbf{4}}}\; \to {\text{ }}{{\mathbf{K}}_{\mathbf{2}}}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{2}}}\left( {\mathbf{s}} \right){\text{ }} + {\text{ }}{{\mathbf{O}}_{\mathbf{2}}}\]
On heating with an alkali, potassium permanganate it produces manganese and oxygen gas is evolved.
\[{\mathbf{4KMn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{4KOH}}\; \to {\text{ }}{\mathbf{4}}{{\mathbf{K}}_{\mathbf{2}}}{\mathbf{Mn}}{{\mathbf{O}}_{\mathbf{4}}}\; + {\text{ }}{\mathbf{2}}{{\mathbf{H}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} + {\text{ }}{{\mathbf{O}}_{\mathbf{2}}}\]
Note: In qualitative analysis, Potassium permanganate is used for determining the permanganate value. It is also used in the treatment of various bacterial infections.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




