
The colour of the \[{X_2}\] molecule of group \[17\] elements changes gradually from yellow to violet down the group. This is due to:
This question has multiple correct options
A. The physical state of \[{X_2}\] at room temperature changes from gas to solid down the group
B. Decrease in \[{\pi ^*} - {\sigma ^*}\] gap down the group
C. Decrease in HOMO – LUMO gap down the group
D. Decrease in ionization energy down the group
Answer
582.3k+ views
Hint: As we know, Group \[17\] elements are nothing but the halogen i.e. fluorine, chlorine, bromine, iodine. Which exist in the diatomic form, and have highest electronegativity. Due to the valence shell electron is p orbital it will show colour due to jumping of electron from ground state to excited state and coming back to the ground state after releasing energy.
Complete answer:
Let us show the MOT Diagram of the group\[17\] of fluorine with HOMO – LUMO gap with \[h\nu \] as energy between them as follows;
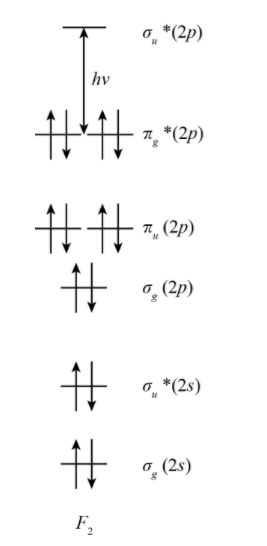
From this diagram, we can see that these gaps will decrease from top to bottom. The origin of the color of the halogens is the excitation between the highest occupied \[\pi *\] Molecular Orbital and the lowest unoccupied \[\sigma *\] Molecular Orbital. The energy gap between the HOMO and LUMO decreases according to \[{F_2}\; > {\rm{ }}C{l_2}\; > {\rm{ }}B{r_2}\; > {\rm{ }}{I_2}\].
The size of the atom will decide the amount of energy required for excitation. The force of attraction between the nucleus and the outer electrons is very large in the Fluorine which is the smallest element in the group. Hence, it will appear pale yellow as it requires a large excitation energy and absorbs violet light (high energy). Whereas, iodine will appear dark violet as it needs significantly less excitation energy and absorbs yellow light of low energy, in the same way it is possible to explain the greenish yellow color of chlorine and the reddish brown color of bromine. The halogens show a variety of colors when dissolved in different solvents can be explained by weak donor-acceptor interaction and charge-transfer bands is due to interaction with the HOMO \[\sigma _u^*\] orbital.
Hence, the correct options are (B) and (C).
Note:
Astatine (As), and Tennessine (Ts) they are not colored compound, because it cannot be explained by weak donor-acceptor interaction and charge-transfer bands is due to interaction with the HOMO \[\sigma _u^*\] orbital.
Complete answer:
Let us show the MOT Diagram of the group\[17\] of fluorine with HOMO – LUMO gap with \[h\nu \] as energy between them as follows;

From this diagram, we can see that these gaps will decrease from top to bottom. The origin of the color of the halogens is the excitation between the highest occupied \[\pi *\] Molecular Orbital and the lowest unoccupied \[\sigma *\] Molecular Orbital. The energy gap between the HOMO and LUMO decreases according to \[{F_2}\; > {\rm{ }}C{l_2}\; > {\rm{ }}B{r_2}\; > {\rm{ }}{I_2}\].
The size of the atom will decide the amount of energy required for excitation. The force of attraction between the nucleus and the outer electrons is very large in the Fluorine which is the smallest element in the group. Hence, it will appear pale yellow as it requires a large excitation energy and absorbs violet light (high energy). Whereas, iodine will appear dark violet as it needs significantly less excitation energy and absorbs yellow light of low energy, in the same way it is possible to explain the greenish yellow color of chlorine and the reddish brown color of bromine. The halogens show a variety of colors when dissolved in different solvents can be explained by weak donor-acceptor interaction and charge-transfer bands is due to interaction with the HOMO \[\sigma _u^*\] orbital.
Hence, the correct options are (B) and (C).
Note:
Astatine (As), and Tennessine (Ts) they are not colored compound, because it cannot be explained by weak donor-acceptor interaction and charge-transfer bands is due to interaction with the HOMO \[\sigma _u^*\] orbital.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




