
The closest-packing sequence ABAB…. represents
A)Primitive cubic packing
B)Body-centred cubic packing
C)Face-centred cubic packing
D)Hexagonal packing
Answer
592.5k+ views
Hint: Other than face-centred cubic structure, hexagonal close packing is also able to achieve the highest packing density of \[74\% \] . It consists of alternating layers of spheres or atoms arranged in a hexagon with one additional atom at the centre. The latest structure is essentially an alternative pattern of layers A and B.
Complete step-by-step answer:
In primitive closed packing, the second row is placed in contact with the first one such that the spheres of the second row are exactly above those of the first row. The spheres of these two rows can be aligned horizontally as well as vertically. Since both the rows are exactly the same, we can call them A type and can write the series as AAA….
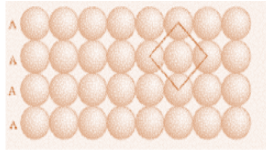
A body centred cubic unit cell has an atom at each of its corners and one atom at its body centre. It has no closed packed planes and therefore does not have a stacking sequence, but it has closed packed directions. We can say that there are six planes of this type and each contains two closed packed divisions.

In cubical closed packed structure or FCC we observe the packing ABCABC…. The first layer A is arranged horizontally below which the second layer B is placed such that the atom of B lies between two atoms of A. The third layer may be placed above the second layer in a manner such that its spheres cover the octahedral voids. The spheres of the third layer are not aligned with those of either the first or second layer. This arrangement is called c type. Only when the fourth layer is placed, its spheres are aligned with those of the first layer and this pattern is observed in FCC molecules.
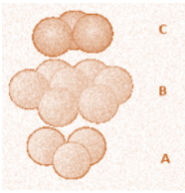
Hexagonal close packing structure is formed when alternate layers of atoms are arranged in a hexagon having an additional atom at the centre. Another layer of atoms is sandwiched between these two hexagonal layers in the form of a triangle and the atoms of this layer fill the tetrahedral holes which are created by the top and bottom layers. We can see the latest structure drawn below. It has an alternative pattern of layers A and B and the series can also be written as ABAB… and so on.

Hence, the correct option is (D).
Note: Since, HCP and FCC have packing efficiency of \[74\% \] which means \[74\% \] space is filled. The remaining space is present in the form of two types of voids-octahedral voids and tetrahedral voids. Other types of packing are not close-packing as they have less packing efficiency. Such as, in BCC \[68\% \] space is filled and in primitive cubic lattice only \[52.4\% \] space is filled.
Complete step-by-step answer:
In primitive closed packing, the second row is placed in contact with the first one such that the spheres of the second row are exactly above those of the first row. The spheres of these two rows can be aligned horizontally as well as vertically. Since both the rows are exactly the same, we can call them A type and can write the series as AAA….

A body centred cubic unit cell has an atom at each of its corners and one atom at its body centre. It has no closed packed planes and therefore does not have a stacking sequence, but it has closed packed directions. We can say that there are six planes of this type and each contains two closed packed divisions.

In cubical closed packed structure or FCC we observe the packing ABCABC…. The first layer A is arranged horizontally below which the second layer B is placed such that the atom of B lies between two atoms of A. The third layer may be placed above the second layer in a manner such that its spheres cover the octahedral voids. The spheres of the third layer are not aligned with those of either the first or second layer. This arrangement is called c type. Only when the fourth layer is placed, its spheres are aligned with those of the first layer and this pattern is observed in FCC molecules.

Hexagonal close packing structure is formed when alternate layers of atoms are arranged in a hexagon having an additional atom at the centre. Another layer of atoms is sandwiched between these two hexagonal layers in the form of a triangle and the atoms of this layer fill the tetrahedral holes which are created by the top and bottom layers. We can see the latest structure drawn below. It has an alternative pattern of layers A and B and the series can also be written as ABAB… and so on.

Hence, the correct option is (D).
Note: Since, HCP and FCC have packing efficiency of \[74\% \] which means \[74\% \] space is filled. The remaining space is present in the form of two types of voids-octahedral voids and tetrahedral voids. Other types of packing are not close-packing as they have less packing efficiency. Such as, in BCC \[68\% \] space is filled and in primitive cubic lattice only \[52.4\% \] space is filled.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




