
The chemical nature of hydrogen peroxide is :
A.An oxidising and reducing agent in the acidic median, but not in the basic medium.
B.An oxidising and reducing agent in both acidic and basic medium.
C.Reducing agent in the basic median, but not in the acidic median.
D.The oxidising agent in acidic medium, but not in basic medium.
Answer
590.7k+ views
Hint: To answer this question, you should recall the properties of hydrogen peroxide. The chemical formula for hydrogen peroxide is ${H_2}{O_2}$. The compounds in which an element is present at an intermediate oxidation state can act as a reducing and oxidizing agent.
Complete step by step answer:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The structure of the molecule can be drawn as:
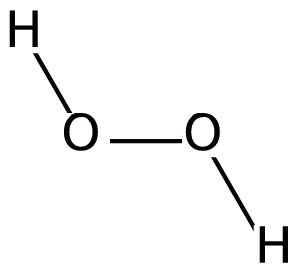
Hydrogen peroxide is a colourless liquid and is used as a bleaching agent and disinfectant due to its property to produce free radicals on decomposition. Hydrogen peroxide decomposes when exposed to sunlight, hence it is stored in wax-lined glass or plastic containers and kept in the dark. This process is catalyzed and accelerated by traces of alkali metals. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. Hydrogen peroxide is both acidic and the basic medium acts as an oxidizing as well as the reducing agent. This is because the oxygen atom in peroxide is present at an intermediate oxidation state of $ - 1$ . So, it can oxidise to zero oxidation state as well as reduce to $ - 2$ oxidation state.
Hence, the correct option is B.
Note:
The preparation of Hydrogen Peroxide:
\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\] is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $an ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Complete step by step answer:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The structure of the molecule can be drawn as:

Hydrogen peroxide is a colourless liquid and is used as a bleaching agent and disinfectant due to its property to produce free radicals on decomposition. Hydrogen peroxide decomposes when exposed to sunlight, hence it is stored in wax-lined glass or plastic containers and kept in the dark. This process is catalyzed and accelerated by traces of alkali metals. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. Hydrogen peroxide is both acidic and the basic medium acts as an oxidizing as well as the reducing agent. This is because the oxygen atom in peroxide is present at an intermediate oxidation state of $ - 1$ . So, it can oxidise to zero oxidation state as well as reduce to $ - 2$ oxidation state.
Hence, the correct option is B.
Note:
The preparation of Hydrogen Peroxide:
\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\] is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $an ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




